

Get access to all handy features included in the IVIS website
- Get unlimited access to books, proceedings and journals.
- Get access to a global catalogue of meetings, on-site and online courses, webinars and educational videos.
- Bookmark your favorite articles in My Library for future reading.
- Save future meetings and courses in My Calendar and My e-Learning.
- Ask authors questions and read what others have to say.
Stomach
Get access to all handy features included in the IVIS website
- Get unlimited access to books, proceedings and journals.
- Get access to a global catalogue of meetings, on-site and online courses, webinars and educational videos.
- Bookmark your favorite articles in My Library for future reading.
- Save future meetings and courses in My Calendar and My e-Learning.
- Ask authors questions and read what others have to say.
Read
Principles of Gastric and Pyloric Surgery
Maria A. Fahie
Introduction
The surgeon should consider specific principles of surgery to produce the best clinical outcome in animals undergoing surgical procedures of the stomach and pylorus. These principles are applicable to surgery involving all of the gastrointestinal tract although only procedures involving the stomach and pylorus will be described in this chapter (Table 19-1).

Anatomy
The stomach has a rich blood supply derived from the celiac artery. Branches of the left and right gastric, splenic and left and right gastroepiploic arteries provide the main blood supply to the stomach (Figure 19-1). Innervation of the stomach is an important anatomical consideration since its disruption intraoperatively could lead to postoperative gastric and biliary tract dysmotility. The primary innervation is parasympathetic, from the vagus nerve. The dorsal and ventral vagus trunks pass through the esophageal hiatus. The ventral trunk branches to supply the lesser curvature, pylorus and liver. The dorsal trunk supplies the lesser curvature, ventral stomach wall, and then follows branches of the celiac and cranial mesenteric arteries. Sympathetic gastric innervation stems from the celiacomesenteric plexus with fibers following the gastric branches of the celiac artery (Figure 19-2). The spinal ganglia responsible for gastric innervation can span from C2 to L5, and peak from T2 to T10.
The cardia of the stomach and pylorus are relatively fixed in place by the esophagus and hepatoduodenal ligament while the mid-portion of the stomach is more mobile.
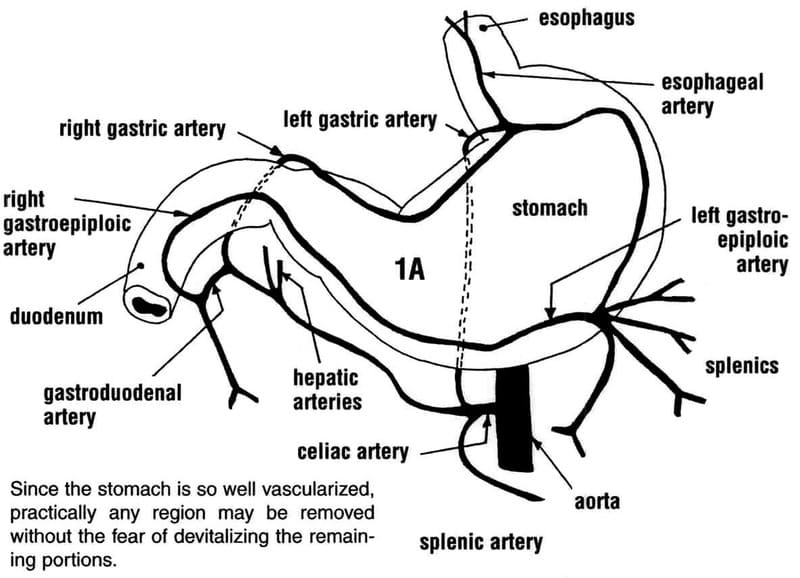
Figure 19-1. Pertinent gastric arterial anatomy. Reprinted with permission from: Anderson S, Gill P, Lippincott L, Somerville M, Shields S, Balfour R, Wilson E. Dimensions in Surgery: Partial Gastrectomy. Pulse (an official publication of the Southern California Veterinary Medical Association): May, 2002.

Figure 19-2. Gastric innervation.
In deep chested dogs, access to the stomach and pylorus can be limited by its omental and mesenteric attachments including the hepatoduodenal ligament, a part of the lesser omentum. This structure contains the hepatic arteries, lymphatics, vagus nerve branches, portal vein and bile duct, therefore its incision is associated with risk of damage to those structures (Figure 19-3). Exposure to the stomach is best enhanced by increasing the length of the abdominal wall incision cranially to the xiphoid cartilage of the sternum, and caudally past the umbilicus. When extending cranially, avoid inadvertent incision of the diaphragm resulting in iatrogenic pneumothorax. Exposure can also be enhanced with devices such as a Balfour abdominal retractor.
Metabolic Abnormalities
Animals that require gastric surgery have clinical signs such as vomiting, anorexia, dehydration, abdominal pain, and gastric distension. Medical management of these clinical signs may be necessary pre and postoperatively and may include antisecretory agents (H2 receptor antagonists), proton pump inhibitors, mucosal protectants, prostaglandin analogues, prokinetic agents and/or antiemetic agents. With acute or chronic vomiting, fluid and electrolyte abnormalities and dehydration are common and should be corrected by intravenous fluid administration prior to anesthesia and surgery if possible. Serum chemistries and electrolyte levels should be obtained prior to surgery. Potassium loss in vomitus and urine can lead to hypokalemia. Hypochloremia can result due to loss of chloride-rich gastric secretions. Hypochloremia can also be exacerbated in hypokalemic patients with reduced renal nephron reabsorption of chloride. The acid-base status of the patient can be normal if loss of gastric hydrochloric acid and bicarbonate-rich duodenal fluid is simultaneous. Metabolic acidosis can be present due to dehydration, prerenal azotemia, and lactic acidosis from compromised tissue perfusion. Hypochloremic metabolic alkalosis indicates severe loss of gastric content and is most indicative of gastric outflow obstruction.
Neoplastic Disease
Dehiscence risk could be higher in patients with neoplasia undergoing gastrotomy for biopsy, although a study of 53 cats with alimentary LSA were not at higher risk.
Prophylactic Antibiotic Administration
Prophylactic antibiotic administration is controversial, since gastric content is generally not high in bacterial numbers in contrast to other regions of the small and large intestine. Opening the gastric lumen is considered a clean-contaminated surgical procedure. Factors that should prompt the surgeon to consider administration of antibiotics include geriatric or debilitated patients, prolonged intraoperative time or compromised aseptic technique. The choice of a specific antimicrobial drug should be based on the organisms within the lumen of the incised organ, or present on the skin at the ventral midline incision site. The duration of antibiotic administration depends upon whether the agent is being administered prophylactically or therapeutically. For prophylactic administration, the antibiotic is given immediately preoperatively (induction of general anesthesia) and continued for the first 12 hours postoperatively. For therapeutic use, the duration of administration should be based upon the pharmacokinetics of the agent used and may extend 10 to 14 days. Cephalexin (22 mg/ kg IV or PO TID) is an easily administered, economical antibiotic that provides an adequate spectrum for most gastric procedures.
Approach
The surgeon should perform complete abdominal exploration prior to all gastric procedures in order to determine the extent of the primary disease process or to identify other disease. A ventral midline celiotomy incision is made, beginning at the xiphoid cartilage cranially and extending at minimum to the region of the umbilical scar caudally. An incision extending more caudally is necessary in some cases to perform complete exploration of all abdominal structures. The falciform ligament is prominent in the cranial aspect of the incision and can be either excised or retracted to aid the surgeon’s visualization of abdominal structures and make closure of the incision more precise. Branches of the internal thoracic and cranial epigastric artery supply the cranial aspect of falciform tissue near the xiphoid and need to be ligated for hemostasis if it is excised. Smaller vascular branches running perpendicular to the linea into the falciform fat can be ligated or cauterized with electrocautery for hemostasis. Moistened laparotomy pads are placed to protect the edges of the abdominal wall, and self-retaining abdominal retractors such as Balfour abdominal retractors or blunt Weitlaner retractors are used to enhance visualization of the abdominal cavity. The surgeon must carefully avoid damage to the bile duct as it traverses the hepatoduodenal ligament (Figure 19-3). The surgeon should examine all abdominal organs and the entire gastrointestinal tract prior to gastrotomy or enterotomy to reduce manipulation of these potentially contaminated tissues within the abdomen.
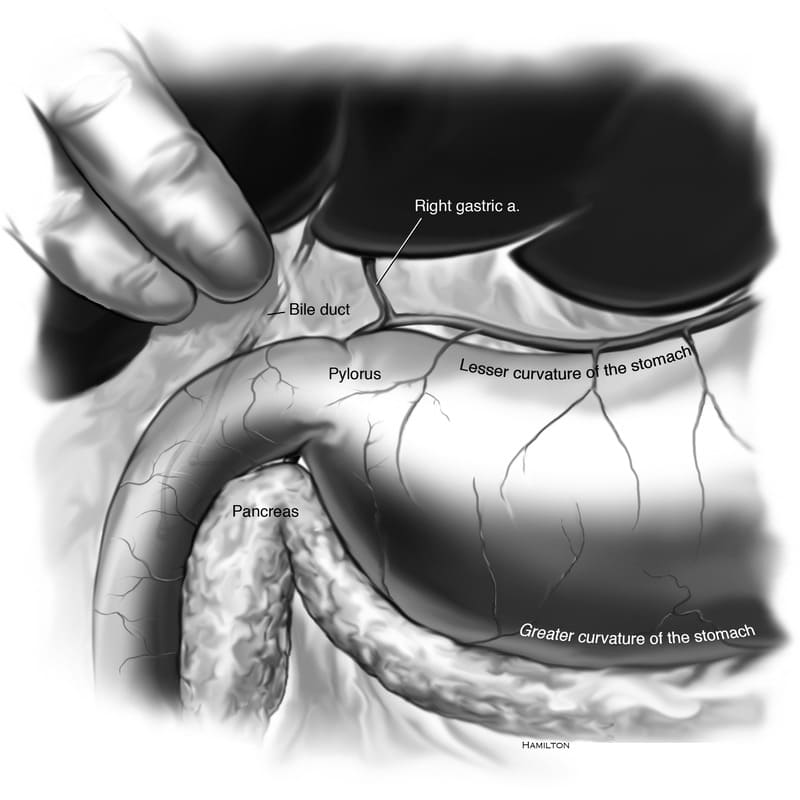
Figure 19-3. Anatomy of the bile duct. It is important to avoid damage to the bile duct as it traverses the hepatoduodenal ligament.
Aseptic Technique
The surgeon may use several techniques to decrease contamination of the abdominal cavity and incision during gastrointestinal surgery. Contamination of the abdomen after gastrotomy can be reduced by double-gloving. The surgeon wears an extra one-half sized larger pair of gloves during gastrointestinal tract surgery and removes the contaminated outer gloves prior to abdominal lavage and closure. Contamination of the abdomen by gastric content is reduced by packing off the stomach from the abdominal cavity with laparotomy pads. The stomach is exteriorized as much as possible from the abdomen by placement of stay sutures or Babcock forceps on the serosal surface. Stay sutures are positioned around proposed gastrointestinal incisions to maintain gentle tissue traction and to aid luminal visualization and prevent spillage of luminal contents. The stay sutures should be placed with a substantial (1cm) full-thickness inclusion of gastric wall to prevent accidental tissue tearing as tissues are manipulated. Frequent reapplication of warm saline to exposed tissues and the laparotomy pads is performed intraoperatively and prevents dessication of tissues. After closure of the gastric wall, contaminated laparotomy pads or sponges are removed and replaced. The abdomen is lavaged with warm saline to remove blood clots, tissue debris and to reduce bacterial numbers. Contaminated instruments are discarded and clean instruments used for abdominal wound closure.
Atraumatic Tissue Handling
Gastric tissues can be friable and atraumatic tissue forceps or an assistant’s fingers induce less tissue trauma than some surgical instruments during tissue manipulation. Surgical instruments that are valuable for gastric surgery include DeBakey tissue forceps, Babcock forceps and Doyen intestinal forceps. As an alternative to Doyen forceps, Allis tissue forceps can be modified by placing moistened gauze sponges around the arms of the instrument to be applied to tissue. The thickness of the gauze determines the amount of pressure applied. Bobby pins can also be sterilized and used as atraumatic intestinal forceps if an assistant’s fingers are not available.
Palpation of Gastric Foreign Bodies
Gastric foreign bodies can be difficult to palpate if there is excessive gastric content and/or if the object is relatively thin, flat and lying against the gastric wall. Gastric contents can be removed by orogastric tube passage and flushing intraoperatively prior to gastrotomy. Alternatively, contents can be carefully removed with suction after gastrotomy, using a Yankaeur or Poole suction tip. If necessary, the surgeon can manually remove gastric content using care to prevent spillage into the abdomen. Gentle and thorough palpation of the entire stomach will permit foreign body location and removal. Palpation of the dorsal gastric surface is aided by digitally creating a fenestration in the greater omentum adjacent and caudal to the greater curvature. If a gastric foreign body is retrieved intraoperatively, the entire intestinal tract should be carefully examined and gently palpated to ensure that no other foreign material is present that could cause an intestinal obstruction. Foreign bodies located in the caudal esophagus can sometimes be removed safely by a gastrotomy incision. Sterile water-soluble lubricant can be used to protect the esophageal and gastric mucosal surfaces during gentle digital palpation and retraction to move the object into the stomach.
Suture Material and Pattern
Many gastric closure techniques are described in the literature including: single-layer appositional or inverting, double layer-inverting or appositional seromuscular (excluding the mucosal layer); and double-layer, appositional and inverting full-thickness closure. The goal of gastric closure is a leak-proof seal with sufficient strength to heal. The submucosal layer, with its abundant collagen, offers the most strength for suture and should be incor- porated in all types of closure. A potential justification for exclusion of the gastric mucosal layer is the potential for some types of suture material to wick gastric fluid to the serosa and peritoneum. An advantage of closure of the mucosal layer is reduced postoperative mucosal hemorrhage into the gastric lumen. The classic closure of a hollow viscus using a double-layer inverting pattern such as a Cushing pattern, followed by a Lembert pattern, is widely used and successful. Inverting closure patterns can be difficult to perform if gastric tissue is thickened, and can compromise lumen diameter compared with appositional patterns. Alternatively, some surgeons prefer to close the mucosa with a simple continuous appositional pattern followed by a Cushing pattern as the 2nd layer of closure. It is recommended that the surgeon apply additional throws to each end of continuous suture patterns (5 to 6 throws of suture) to ensure knot stability.
The ideal suture material for gastric closure is monofilament, absorbable [poliglecaprone 25-MonocrylTM (Ethicon, Somerville, NJ), glycomer 631-BiosynTM (Syneture/Covidien, Norwalk, CT), polydioxanone-PDS IITM (Ethicon, Somerville, NJ) or polytrimethylene carbonate-MaxonTM (Davis & Geck, Chicago, IL)] with a tapered needle, usually 2-0 to 4-0 in size. Based on the speed of gastric tissue healing, there is little justification for use of non-absorbable suture materials. Pyloric obstruction following closure of a gastrotomy incision with polypropylene has been described. Recent studies of absorbable knotless barbed suture material demonstrated comparable bursting strength and closure time for gastrotomy.
Stapling Devices
Stapling devices provide a stronger anastomosis with shorter surgical time compared to hand suturing, however their use may be cost-prohibitive for some patients and procedures. Stapling devices should be considered for partial gastrectomy and/ or gastroduodenostomy (Billroth I) diversion procedures. The linear thoracoabdominal (TATM 30, 55, 90; (USSC, Tyco Healthcare Group LP, Norwalk, CT) and gastrointestinal anastomosis (GIATM 60, 80; USSC, Tyco Healthcare Group LP, Norwalk, CT) are the most versatile stapling devices for gastric procedures. The TA applies a double staggered row of staples. The GIA applies two double staggered rows of staples and cuts between them.
Omental Patching
The potential benefit of enhanced vascular supply to healing gastric tissue is realized. In most cases, the omentum can be easily advanced over the incision site and tacked to the surrounding gastric serosa with a few simple interrupted sutures of monofilament absorbable.
Abdominal Lavage
Abdominal lavage is indicated following gastric procedures to dilute and/or remove any gastric content spillage. Any gastric incision sites should be lavaged locally after closure, while the stomach is still exteriorized from the abdomen. Prior to abdominal incision closure, copious abdominal irrigation is recommended with warm, isotonic, crystalloid solution such as 0.9% saline or lactated ringer’s solution. Lavage should continue until the fluid collected is clear. The preferred temperature of lavage fluid should approximate normal body temperature 38.5°C (101.3°F). In hypothermic patients, fluid at 43°C (110°F) successfully increased body temperature during 2 to 6 minutes of lavage.
Postoperative Pain Management
Appropriate postoperative pain management is being recognized as a key factor in the successful recovery of surgical patients. In gastric surgery patients, nonsteroidal antiinflammatory agents and morphine derivative agents may have undesirable effects on gastric mucosa and gastrointestinal tract motility. Tramadol hydrochloride 50 mg (Tramadol®, 1-2 mg/kg PO-TID, Mutual Pharmaceutical Co. Inc., Philadelphia, PA) may provide necessary pain relief without the side effects discussed above. Maropitant, a neurokinin receptor antagonist and substance P blocker, may provide pain relief and anti-emetic benefits. (CereniaTM, 2-8 mg/kg PO once daily for up to 5 days or injectable, Zoetis, Florham Park, NJ). Gabapentin, an anticonvulsant and analgesic through incompletely understood mechanisms, may also be well tolerated. (NeurontinTM, 10-20 mg/kg PO once to twice daily, Pfizer, New York, NY). The proper combination of medications, light activity and appropriate nutrition is necessary for a gradual return to normal function.
Postoperative Alimentation and Nutrition
The benefit of early introduction of nutrients on wound healing is realized. Complications including mucosal atrophy, ileus, and sepsis from bacterial translocation through the intestinal wall are all associated with malnutrition. Decisions regarding diet choice and initiation of oral alimentation must be made considering the preoperative condition, intraoperative findings and surgical procedures performed. Auscultation of gut sounds within 24 hours postoperatively is a positive indication that gastrointestinal motility is resuming. For a simple gastrotomy patient, oral alimentation can begin with water and/or a bland gruel within 24 hours postoperatively, provided no vomiting has been noted. Some patients undergoing gastric surgery might require an alternate route for alimentation postoperatively. Ultimately, this should be anticipated preoperatively, so that the feeding tube placement can occur simultaneously with surgery. In order to bypass the stomach, a jejunostomy tube might be indicated. Research suggests that intrajejunal nutrition enhances the gastrointestinal tract barrier and does not exacerbate pancreatitis. Intravenous total parenteral nutrition is another option but not as successful to prevent mucosal atrophy or increased collagen deposition. Supplementation of vitamin B12 (cyanocobalamin injection) should be considered in gastrectomy and gastroduodenostomy patients, since deficiency and anemia could result. The mechanism underlying the deficiency is the reduction in numbers of parietal cells to secrete intrinsic factor, which is crucial for vitamin B12 to complex with and become absorbed in the intestine.
Suggested Readings
Bright RM, Jenkins C, DeNovo RC. Pyloric obstruction in a dog related to gastrotomy incision closed with polypropylene. J Small Anim Pract 1994; 35 (12): 629-632.
Clark GN. Gastric surgery with surgical stapling instruments. Vet Clin North Am Small Anim Pract 1994;24:279-304.
Clark GN, Pavletic MM. Partial gastrectomy with an automatic stapling instrument for treatment of gastric necrosis secondary to gastric dilatation-volvulus. Vet Surg 1991: Jan-Feb; 20(1):61-8.
Coolman BR, Ehrhart N, Marretta SM. Use of skin staples for rapid closure of gastrointestinal incisions in the treatment of canine linear foreign bodies. J Am Anim Hosp Assoc. 2000 Nov-Dec;36(6):542-7.
Radlinsky MG. Digestive System. In: Fossum, TW ed. Small Animal Surgery, 4th ed. WB Saunders, Philadelphia, 2013, pp 461-497.
Khurana RK, Petras JM. Sensory innervation of the canine esophagus, stomach and duodenum. Am J Anat 1991,192: 293-306.
Nawrocki MA, McLaughlin R, Hendrix PK. The effects of heated and room temperature abdominal lavage solution on core body temperature in dogs undergoing celiotomy. J Am Anim Hosp Assoc, 41, 1, 61-67, 2005.
Qin HL, Su ZD, Hu LG, et al. Effect of early intrajejunal nutrition on pancreatic pathological features and gut barrier function in dogs with acute pancreatitis. Clinical Nutrition, 21, 6, 2002 469-473.
Cornell K. Stomach. In: Tobias K, Johnston S eds. Veterinary Surgery Small Animal. WB Saunders, Philadelphia, 2012, pp 1484-1512.
Ross WE, Pardo AD. Evaluation of an omental pedicle extension technique in the dog. Vet Surg, 22, 1, 37-43, 1993.
Seim III HB, Bartges JW. Enteral and Parenteral Nutrition. In, Handbook of Small Animal Gastroenterology (2nd ed), 2003, 416-462.
Smith MM, Waldron DR. Approach to the Stomach and Approach to the Pylorus.In:AtlasofApproachesforGeneralSurgeryoftheDogandCat. Philadelphia: WB Saunders, 1993, 184-189.
Tsukamoto M, Enjoji A, Ura K, Kanematsu T. Preserved extrinsic neural connection between gall bladder and residual stomach is essential to prevent dysmotility of gall bladder after distal gastrectomy. Neurogastroenterol Mot 2000, 12: 23-31.
Ehrhart NP, Kaminskaya K, Miller JA, Zaruby JF. In vivo assessment of absorbable knotless barbed suture for single layer gastrotomy and enterotomy closure. Vet Surg 42 (2013) 210-216.
Ellison GW. Complications of gastrointestinal surgery in companion animals. Vet Clin Small Anim 41 (2011) 915-934.
Smith AL, Wilson AP, Hardie RJ, Krick EL, Schmiedt CW. Perioperative complications after full-thickness gastrointestinal surgery in cats with alimentary lymphoma. Vet Surg 40 (2011) 849-852.
Gastrotomy
Maria A. Fahie
Indications
The most common indication for gastrotomy is for identification and removal of suspected foreign bodies or for gastric biopsy. Postoperative peritonitis and stricture are rare in simple gastrotomy patients.
In medium to large patients, with relatively small and smooth gastric foreign bodies, removal can occur with induction of vomiting. Alternatively, gastric lavage or endoscopy can be performed with general anesthesia. A recent retrospective study of 102 dogs undergoing endoscopic removal of esophageal and gastric foreign bodies (FB) concluded a low complication rate provided patients were > 10kg and did not have sharp bone FB or FB present for more than 3 days.
Surgical Technique
The optimal location for gastrotomy is the fundus region, avoiding branches of the left and right gastric and gastroepiploic arteries and associated nerves. Babcock forceps or stay sutures are placed 1-2 cm from each end of the planned gastrotomy site and used to maintain tissue tension to facilitate the incision (Figure 19-4). A scalpel blade (No. 10, 11, or 15) is used to make a controlled full-thickness stab incision. Alternatively, the scalpel blade can be used to make a partial-thickness incision through the serosa, muscularis and submucosa, and the mucosal incision performed alone using a similar blade technique. Metzenbaum scissors can be used to extend the stab incision as indicated in the individual case. Hemostasis is achieved using hemostats or electrocautery. Gastric contents are removed as needed in order to retrieve the foreign body or identify the lesion that prompted the gastrotomy. If a linear foreign body is identified, the section within the stomach should not be detached until the distal extent within the small intestine has been identified via enterotomy. Gastric biopsy is indicated in all cases requiring gastrotomy, whether or not gross abnormalities are detected. A full-thickness strip of tissue can be excised along the gastrotomy incision and submitted for histopathologic analysis.
Closure
Prior to closure, all potentially contaminated instruments, suture material, sponges, drapes and gloves are discarded and replaced. In general, I prefer a two layer gastric closure with a simple continuous appositional pattern using monofilament absorbable suture material usually 2-0 or 3-0 in size. The first layer incorporates the mucosa and the second layer incorporates the submucosa, muscularis and serosa. If there is concern that suture material exposed within the gastric lumen can wick fluid or contaminants into the gastrotomy incision or peritoneal space, then another closure pattern should be chosen. A double layer inverting pattern, such as continuous Cushing or Lembert, can be placed incorporating only the submucosa, muscularis and serosa (Figure 19-4).
Alternatively, stapling devices such as disposable skin staples (4.8 mm by 3.4 mm), or a linear stapling device (TATM) can be used. The primary advantage is reduction in operative time and strength of tissue apposition compared with hand suturing techniques. The primary disadvantage is cost and availability of stapling equipment. Considering these factors, the benefit of staplers is probably limited to larger gastrotomy incisions. The skin stapling technique is described as a double-layer closure, with mucosa/ submucosa apposed using a simple continuous pattern of monofilament, absorbable suture material, and serosa/muscularis apposed using skin staples placed at 3 mm intervals facilitated by traction on stay sutures at each end of the incision. A TATM can be applied to the gastrotomy site, providing an everted closure of all tissue layers at once, with a double staggered row of staples.

Figure 19-4. Gastrotomy. A. Stay sutures allow gentle tissue traction. An inverted #10, 11 or 15 blade is used to make a full-thickness stab incision into the stomach. A partial thickness initial incision is also acceptable. B. Extension of the incision with Metzenbaum scissors. C. Single-layer closure using a simple continuous appositional pattern incorporating mucosa, submucosa, muscularis and serosa simultaneously. D. Alternatively, a double-layer closure can be performed with the initial step of mucosal apposition using a simple continuous pattern. E. The second step is submucosal, muscularis and serosal apposition using a simple continuous suture pattern. F. Inverting Lembert pattern. G. Inverting Cushing pattern.
Suggested Readings
Gianella P, Pfammatter NS, Burgener IA. Oesophageal and gastric endoscopic foreign body removal: complications and follow-up of 102 dogs. Journal of Small Animal Practice 2009, 50: 649-654.
Partial Gastrectomy
(Full-Thickness)
Maria A. Fahie
Indications
Benign gastric neoplasia, such as adenomatous polyps or leiomyoma, can be excised via partial full-thickness gastrectomy. Gastric ulcers that are not amenable to medical management can be excised via partial gastrectomy. Malignant gastric neoplasia (adenocarcinoma, leiomyosarcoma, lymphosarcoma and fibrosarcoma) has often metastasized to local lymph nodes, liver or lungs by the time of diagnosis. Partial gastric resection can only be considered palliative in these patients, since long term prognosis is guarded to grave. Up to 75% of the gastric fundus can be removed without significantly affecting food passage.
Surgical Technique
Abdominal exploration is always indicated prior to partial gastrectomy to evaluate the extent or presence of metastatic or other disease processes. The gastric abnormality to be resected is identified by visualization and palpation and an appropriate surrounding margin of grossly normal tissue is planned. Stay sutures facilitate exposure and manipulation. The vascular supply to the region will include branches of the left and right gastric and gastroepiploic vessels depending on the location of the lesion. Those branches supplying the area to be resected are identified and ligated. The stomach has extensive collateral circulation, therefore ligation of the vascular supply to the region of abnormal tissue can generally be performed without compromise to remaining gastric tissue. Atraumatic intestinal forceps, stay sutures, or assistant’s fingers can be used to isolate the tissue that will be remaining. Carmalt forceps can be placed along the margin of the tissue to be resected in order to prevent abdominal contamination with gastric contents. The tissue is incised, removed and submitted for histopathologic analysis.
Closure
For lesions of the lesser curvature, closure of the remaining defect can be performed with hand suturing or stapling techniques similar to those described in the preceding gastrotomy section. Closure of U-shaped defects is facilitated by suturing the appropriate sequence of tissue layers, beginning with the serosal, muscularis and submucosal layers of the dorsal surface of the stomach first which is the deepest layer intraoperatively. Next, the mucosal layer of the dorsal surface, continued to the mucosal layer of the ventral surface. Finally, the serosal, muscularis and submucosal layers of the ventral surface of the stomach. (Figure 19-5A). For lesions of the greater curvature, closure can be performed by hand-suturing similar to as described for gastrotomy. Alternatively, a linear stapling device can be employed (Figure 19-5B). A Carmalt forcep should be placed on the tissue to be resected prior to excision to prevent gastric spillage. An omental patch can be anchored to the stapling site, simply by mobilizing some omental adipose tissue and suturing it to the gastric serosa with several simple interrupted sutures. A GIATM (USSC, Tyco Healthcare Group LP, Norwalk, CT) stapling device could also be used (Figure 19-5C). This device incises and applies a double layer staggered staple line on each side of the incision. The primary advantage is reduced risk of gastric spillage since the resected tissue also has a double row of staggered staples.


Figure 19-5. A. Hand suturing for closure of partial gastrectomy of lesser curvature. B. Linear stapling device for closure of partial gastrectomy of greater curvature.
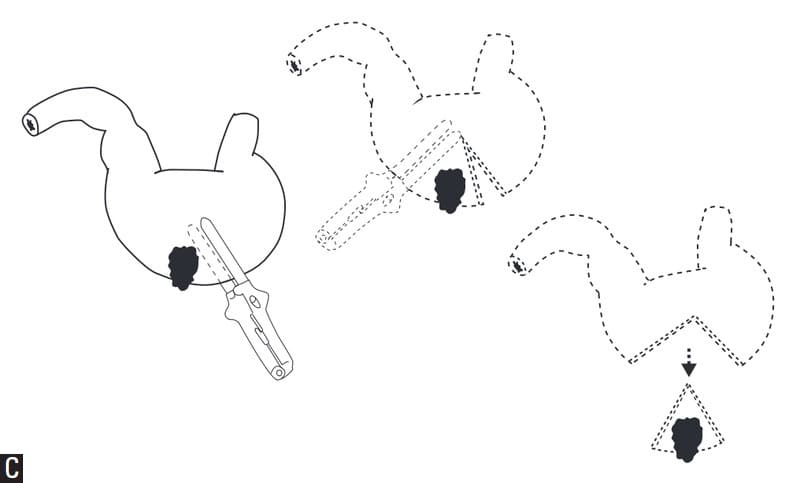
Figure 19-5C. GIA stapling device for closure of partial gastrectomy on greater curvature. Figures 19-5A-C reprinted with permission from: Anderson S, Gill P, Lippincott L, Somerville M, Shields S, Balfour R, Wilson E. Dimensions in Surgery: Partial Gastrectomy. Pulse (an official publication of the Southern California Veterinary Medical Association): May, 2002.
Suggested Readings
Tobias KM. Surgical stapling devices in veterinary medicine: A review. Vet Surg 36 (2007) 341-349.
Partial-Thickness Resection via Gastrotomy Incision
Maria A. Fahie
Indications
This procedure is indicated for mobile, sessile or pedunculated mucosal masses in the cardia or fundus regions.
Surgical Technique
The stomach is exteriorized and isolated from the remainder of the abdominal contents with moistened laparotomy pads. Stay sutures are placed 1 to 2 cm from the ends of the planned gastrotomy. The gastrotomy incision is made in the body of the fundus, directly opposite the mass and midway between the greater and lesser curvature, avoiding gastric arteries and associated nerves (Figure 19-6). The mass is located and a stay suture is placed within it to allow application of traction and to facilitate transection of the surrounding mucosa and submucosa.
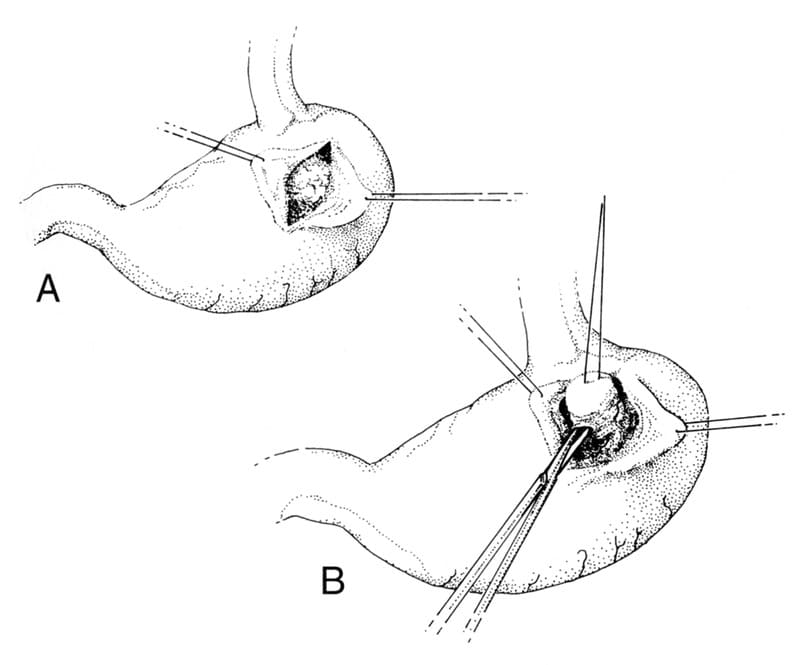
Figure 19-6. Partial thickness submucosal resection of midbody and cardia gastric lesions via gastrotomy. A. The gastrotomy incision is made in the fundic region avoiding gastric arteries and nerves. Stay sutures are placed to facilitate manipulation of the incision and avoid spillage of gastric contents. B. A stay suture is placed within the mass and traction is applied to allow transection of the surrounding mucosa and submucosa. Closure of the remaining mucosal/submucosal defect should begin prior to complete transection of the mass. A simple continuous appositional or inverting pattern is appropriate.
Closure
The mucosal closure is initiated prior to completion of transection of the mass, and performed in stages as the mass is gradually transected. A simple continuous appositional or inverting pattern with monofilament absorbable 3-0 or 4-0 suture material is appropriate. The gastrotomy incision is closed as described previously.
Suggested Readings
Kerpsack SJ, Birchard SJ. Removal of leiomyomas and other noninvasive masses from the cardiac region of the stomach. J Am Anim Hosp Assoc. 1994: Sept/Oct, 30; 500-504.
Swann HM, Holt DE. Canine gastric adenocarcinoma and leiomyosarcoma: a retrospective study of 21 cases (1986-1999) and literature review. J Am Anim Hosp Assoc 2002 Mar-Apr;38(2):157-64.
Y-U Antral Flap Pyloroplasty
Maria A. Fahie
Indications
Delayed gastric emptying necessitates medical management with dietary and prokinetic therapy. In some cases, pyloroplasty is indicated in patients with gastric outflow obstruction and delayed gastric emptying due to congenital or acquired pyloric stenosis from benign proliferative disease of antral and pyloric mucosa (chronic hypertrophic pyloric gastropathy). Breeds with a predisposition to this congenital condition include some brachycephalic dogs (English bulldog, Boston terrier, Boxer) and the Siamese cat. Patients with acquired disease are usually middle-aged or geriatric small breeds such as the Lhasa apso, Shih tzu and Maltese. A study of 45 primarily geriatric patients demonstrated an 85% good to excellent response to surgical management of their hypertrophic disease.
Other intramural causes of acquired lesions affecting gastric outflow include neoplasia, foreign body, hypertrophic or eosinophilic gastritis and antral polyps. Extramural lesions of the liver or pancreas can also compress the pyloric region and affect gastric outflow. Pyloroplasty would not be recommended in those patients with malignant, inflammatory or extramural disease.
Pyloroplasty involves a full-thickness incision and reorientation of the pyloric tissue performed to increase the diameter of the gastric outflow tract. I recommend the Y-U antral flap pyloroplasty procedure, instead of pyloromyotomy (Fredet-Ramstedt) or transverse pyloroplasty (Heineke-Mikulicz), since the Y-U pyloroplasty allows for more resection of hypertrophied pyloric mucosa while significantly expanding the diameter of the gastric outflow tract and decreasing gastric emptying time.
Diagnostics to confirm gastric outflow obstruction are crucial and should include contrast radiographs, abdominal ultrasound and gastroduodenoscopy. Retention of a barium meal in the stomach can be difficult to interpret on contrast radiographic studies, since there is a wide range of gastric emptying times reported in normal dogs (5 to 15 hours). Generally, retention > 8-10 hours is considered prolonged and indicative of gastric outflow obstruction. Abdominal ultrasound can identify intramural submucosal/muscularis abnormalities not necessarily visible on radiographs or with endoscopy. Gastroduodenoscopy provides further detail regarding mucosal causes of pyloric obstruction. If pyloroplasty is performed in a patient without diagnostically confirmed gastric outflow obstruction, the procedure can actually cause delayed gastric emptying by overstimulation of the enterogastric reflex from the early passage of hyperosmolar gastric content into the duodenum. Diagnosis of delayed gastric emptying provides the greatest challenge to the surgeon. A review of diagnostic techniques is recommended.
Surgical Technique
The pylorus and pyloric antrum are identified and isolated using a combination of moistened laparotomy sponges and stay sutures. The pyloric ring is identified by palpation. Using a #10 scalpel blade, a “Y” shaped incision is made in the serosa with the base of the “Y” (Figure 19-7A) just oral to the pyloric ring, and each arm of the “Y” being 3 to 5 cm in length. The incision is extended into the gastric lumen through an initial stab incision with the scalpel blade, and extension with scissors. To facilitate advancement of the antral flap, and alleviate continued obstruction from proliferative pyloric mucosa, a rectangular shaped segment of the exposed hypertrophied pyloric ring tissue can be elevated submucosally, from the portion of the pylorus that is exposed by your incision, but not from the flap to avoid disruption of its vascular supply. Excised tissue can be submitted for histopathology. The muscularis and serosal layers (Figure 19-7B) remain intact, and the hypertrophied mucosal/ submucosal layers (between e & f) are excised.
Closure
The mucosal/submucosal edges remaining after excision of proliferative tissue (e and f) are apposed in a simple continuous suture pattern with monofilament 3-0 or 4-0 absorbable material. The “Y” shaped incision is then sutured closed to form a “U” shaped incision (Figure 19-7C). Care should be taken to contour the tip of the flap to a “U” shape rather than a pointed “V” shape, since the vascular supply to the point may not be adequate. The most distal suture should be placed initially, to ensure proper flap advancement and placement. The remainder of the tissue can be closed using simple interrupted or continuous appositional sutures, both incorporating all 4 tissue layers (mucosa, submucosa, muscularis, serosa) simultaneously.
Postoperative Care
Appropriate postoperative management depends on the patient’s preoperative status. Intravenous fluids should be chosen based on the patient’s hydration status, electrolyte levels and acid-base status, and continued postoperatively until adequate oral alimentation is possible. Medical management of vomiting may be indicated. If there is no vomiting, a low-fat diet can be initiated on the first postoperative day to enhance gastric emptying.
Figure 19-7. Y-U Antral Flap Pyloroplasty. A. The base of the “Y” incision extends slightly onto the stomach side of the pyloric ring (1-2). Each limb of the “Y” (1-2, 2-3, 2-4) is approximately 3 to 5 cm in length. B. a= sub-serosa, b=muscularis, c=submucosa, d=mucosa, e+f= proliferative tissue located in strip between these two letters; Pyloric submucosal resection of hypertrophied mucosal tissue. C. The pyloroplasty incision is closed by advancing the antral flap toward the duodenum, suturing tissue in region #2 to that of region #1. Tissue apposition can be performed using a simple continuous or simple interrupted approximating suture pattern.
Suggested Readings
Allen FJ, Guilford WG, Robertson IG, Jones BR. Gastric emptying of solid radiopaque markers in healthy dogs. Veterinary Radiology and Ultrasound 1996: 37;5:336-344.
Arnbjerg J. Gastric emptying time in the dog and cat. J Am Anim Hosp Assoc 1992: Jan-Feb(28):77-81.
Bright RM, Toal R, Denovo RC, McCracken M, McLauren JB. Effects of the Y-U pyloroplasty on gastric emptying and duodenogastric reflux in the dog. Vet 16 (1987) 392-397.
Burns J, Fox SM. The use of a barium meal to evaluate total gastric emptying time in the Dog. Vet Radiol 1986:27(6):169-72.
Matthiesen DT, Walter MC. Surgical Treatment of chronic hypertrophic pyloric gastropathy in 45 dogs. J Am Anim Hosp Assoc 1986:Mar/ Apr:22:241-247.
Miyabayashi T, Morgan JP. Gastric emptying in the normal dog. A contrast radiographic Technique. Vet Radio 1984;25(4):187-91.
Rivers BJ, Walter PA, Johnston GR, Feeney DA, Hardy RM. Canine gastric neoplasia: Utility of ultrasonography in diagnosis. J Am Anim Hosp Assoc 1997;33:144-55.
Stanton ME, Bright RM, Toal R, DeNovo RC, McCracken M, McLauren JB. Effects of the Y-U pyloroplasty on gastric emptying and duodenogastric reflux in the dog. Vet Surg 1987;16(5):392-7.
Matthiesen DT, Walter MC. Surgical treatment of chronic hypertrophic pyloric gastropathy in 45 dogs. J Am Anim Hosp 1986; Mar-Apr (22): 241-247.
Papageorges M, Breton L, Bonneau NH. Gastric Drainage Procedures: Effects in normal dogs I. Introduction and description of surgical procedures. Veterinary Surgery 1987: 16;5:327-331.
Papageorges M, Breton L, Bonneau NH. Gastric Drainage Procedures: Effects in normal dogs II. Clinical observations and gastric emptying. Veterinary Surgery 1987: 16;5:332-340.
Papageorges M, Breton L, Bonneau NH. Gastric Drainage Procedures: Effects in normal dogs III. Postmortem evaluation. Veterinary Surgery 1987: 16;5:341-345.
Sanchez-Margallo FM, Soria-Galvez F, Ezquerra-Calvo LJ, Uson-Gargallo J. Comparison of ultrasonographic characteristics of the gastroduodenal junction during pyloroplasty performed laparoscopically or via conventional abdominal surgery in dogs. AJVR 64 (2003) 1099-1104.
Wyse CA, McLellan J, Dickie AM, et al. A review of methods for assessment of the rate of gastric emptying in the dog and cat: 1898-2002. J Vet Intern Med 2003:17:609-621.
Billroth I (Gastroduodenostomy)
Maria A. Fahie
Indications
Patients with gastric outflow obstruction due to malignant or inflammatory disease (such as adenocarcinoma or severe gastric ulceration) are candidates for pyloric resection and gastroduodenostomy. However, in a review of 24 dogs undergoing pylorectomy with gastroduodenostomy, median survival time with malignant neoplasia was only 33 days. Preoperative weight loss and malignant neoplasia are associated with shortened survival. Hypoalbuminemia and anemia occurred postoperatively in about 62 and 58% of dogs respectively. The goal of gastroduodenostomy is removal of the entire pylorus without disruption of surrounding structures including the extrahepatic biliary tree, or the biliary and pancreatic duct apertures at the major duodenal papilla. If this is not possible, a gastroduodenostomy procedure should not be performed. Gastrojejunostomy (Billroth II) with cholecystoduodenostomy and possible pancreatic enzyme replacement would be necessary for reconstruction after such an extensive resection. The gastroduodenostomy (Billroth I) requires less diversion from normal physiologic conditions compared with gastrojejunostomy (Billroth II) and as a result, there are fewer long term potential complications. In humans, there are fewer problems with gastritis, pancreatic function impairment and lower esophageal sphincter impairment in patients having gastroduodenostomy compared to those having gastrojejunostomy. In a study of 21 dogs having gastroduodenostomy for resection of adenocarcinoma and leiomyosarcoma, postoperative survival ranged from 3 days to 10 months due to recurrence of preoperative clinical signs. A recent retrospective study indicated that preoperative weight loss and diagnosis of malignant neoplasia were significant risk factors that shortened survival time post pylorectomy and gastroduodenostomy. Hypoalbuminemia and anemia commonly contributed to postoperative morbidity.
Approach and Asepsis
In performing gastroduodenostomy, the approach and aseptic technique are similar to that described in the preceding gastrotomy section. It is crucial to identify and avoid the common bile duct prior to ligation or transection of any structures (See Figure 19-3). Division of the duodenocolic ligament will enable cranial displacement of the caudal duodenal flexure and facilitate approximation of the duodenum and stomach for gastroduodenostomy post-resection of affected tissues.
Surgical Technique
It is important to perform a complete abdominal exploratory to determine the extent of disease prior to gastric diversion procedures. The affected gastric region is identified by visualization and palpation. A surrounding margin of grossly normal tissue is planned. A minimum of 1 cm of duodenum must be maintained orad to the major duodenal papilla in order to avoid postoperative bile and pancreatic duct obstruction. The gastroduodenal artery, biliary tract, hepatic arteries and pancreas must be identified and avoided during manipulations. The vascular supply to the affected region is ligated in a fashion similar to that for a partial gastrectomy procedure (Figure 19-5). Atraumatic forceps are placed on the gastric and duodenal tissue that will remain. Carmalt forceps can be placed on the tissue to be resected. The pylorus is resected and submitted for histopathologic analysis.
Figure 19-8. Gastroduodenostomy (Billroth I)- Hand suturing A. Arteries to be ligated are right gastric (A) and right gastroepiploic (B); avoid the gastroduodenal artery (C). B. Excision of pyloric sphincter and canal. C. Apposition of gastric mucosa in simple continuous or inverting Cushing or Lembert pattern. D. Apposition of gastric seromuscular layers in simple continuous or inverting Cushing or Lembert suture pattern. E. Completed anastomosis between stomach and duodenum.

Figure 19-9. A. Technique for side-to-end gastroduodenostomy (Billroth I) using TA and EEA stapling devices to form a circular anastomosis. After ligation and division of omental vessels, the gastric pouch is closed using an appropriate size thoracoabdominal instrument. The instrument is placed proximal (oral) to the mass, leaving appropriate margins of grossly normal tissue and at least 1cm oral to the major duodenal papilla. Tissue forceps are placed adjacent to the mass, and the stomach is transected with a scalpel blade, using the instrument edge as a cutting guide. B. The pursestring instrument (Furniss clamp) is placed around the duodenum, distal (aboral) to the mass, leaving appropriate margins of grossly normal tissue. Monofilament 3-0 suture is passed through the superior jaw of the pursestring instrument and returned through the inferior jaw. Tissue forceps are placed adjacent to the mass, and the duodenum is transected using the pursestring instrument edge as a cutting guide. C. The pursestring instrument is removed and the appropriately sized end-to-end anastomosis (EEA) cartridge is chosen on the basis of the luminal diameter of the proximal duodenum. A stab incision is made in an avascular portion of the ventral aspect of the stomach, approximately 3 cm away from the edge of the TA staple line. Stay sutures aid in the retraction of the stomach. D. The EEA instrument is introduced, without the anvil, through the stab wound on the ventral surface of the stomach. The center rod of the instrument is exited through a small stab incision in the center of a pursestring suture that has been placed on the dorsal surface of the stomach. The pursestring suture is tied on the stomach side, and the anvil is placed on the central rod. The anvil is introduced into the duodenal lumen and the pursestring suture is tied. The EEA instrument is then closed and fired. A circular, double-staggered row of staples joins the organs, and the circular blade in the instrument cuts a stoma. E. Completion of the gastroduodenostomy. The EEA instrument has been gently removed from the entry site on the ventral surface of the stomach and the staple line inspected for hemostasis. The gastrotomy incision has been closed with a TA instrument. The completed anastomosis consists of two linear staple closures on the stomach and a circular stapled anastomosis forming the gastroduodenostomy.
Closure
Once abnormal gastric/pyloric tissue is resected, the incisions of the remaining gastric and duodenal segments are anastomosed using hand suturing or stapling devices such as a GIATM (gastrointestinal anastomosis) (USSC, Tyco Healthcare Group LP, Norwalk, CT), or TA (thoraco-abdominal) 30, 55, or 90TM (USSC, Tyco Healthcare Group LP, Norwalk, CT) depending on the dimensions. When hand suturing, an end-to-end gastroduodenostomy anastomosis is most simple (Figure 19-8). If there is significant disparity between the gastric and duodenal lumen diameters, the antimesenteric border of the duodenum can be incised longitudinally (“fish mouthed”) to increase its lumen diameter. The anastomosis should be created to resemble the normal anatomic position of the duodenum in relation to the remaining gastric fundus as much as possible, rather than creating a potential blind sac. Gastric mucosal closure is initiated at the margin of the lesser curvature in either a single-layer simple continuous appositional pattern (incorporating mucosa, submucosa, muscularis and serosa) or a double-layer simple continuous appositional pattern (incorporating mucosa first, then the other 3 layers), both with 2-0 to 4-0 monofilament absorbable material depending on patient size. Once the remaining gastric mucosa is a diameter approximating the normal pylorus, full-thickness simple interrupted or continuous, appositional sutures are used to anastomose the duodenum and stomach. The anastomotic site can be tested for leakage by temporarily occluding the tissue on either side of the site and injecting warm saline solution with a 22G needle and syringe. An omental patch can be anchored over the anastomotic site using a few simple interrupted sutures to anchor omentum to the gastric and duodenal serosa.
Alternatively, stapling devices can be used to create a side- to-end gastroduodenostomy with the dorsal surface of the stomach (Figure 19-9). The pyloric antral margin is closed using a linear stapling device of appropriate size. A purse-string suture is placed in the remaining duodenal margin. A small gastrotomy incision is made (using a scalpel blade or GIATM stapling device) to allow access of the EEATM (USSC, Tyco Healthcare Group LP, Norwalk, CT) (end-to-end anastomosis) stapling device. A purse-string suture is placed at the proposed anastomotic site on the dorsal gastric wall and a second gastrotomy incision is performed to allow placement of the EEATM circular stapling device center rod. The anvil is attached to the center rod and introduced into the duodenal segment. Both purse strings are tied. The EEA is fired, released and removed. The result is a gastroduodenostomy with a staggered row of staples that incorporated the second gastrotomy site. The gastrotomy site used for access of the EEA to the stomach is closed using a TA stapling device of appropriate size.
Postoperative Care
Since the pancreas is in close proximity to the tissues being manipulated, postoperative pancreatitis is possible. Refer to the preceding section on postoperative alimentation and nutrition. Appropriate intravenous fluids and medical management are indicated if vomiting occurs.
Suggested Readings
Buhner S, Ehrlein HJ, Thomas G, Schumpelick V. Effects of nutrients on gastrointestinal motility and gastric emptying after Billroth-I gastrectomy in dogs. Dig Dis Sci 1988 Jul; 33(7):784-94.
Buhner S, Ehrlein HJ, Thomas G, Schumpelick V. Canine motility and gastric emptying after subtotal gastrectomy. Am J Surg.1988 Sep;156(3 Pt 1):194-200.
Eisele J, Kovak McClaren J, Runge J J, et al. Evaluation of risk factors for morbidity and mortality after pylorectomy and gastroduodenostomy. Vet Surg 2010, 39: 261-267.
Hunt G. Bilroth 1 or Bilroth 2: To Do or Not To Do? Proceedings of the American College of Veterinary Surgeons, 2005 p201-4.
Sumner AE, Chin MM, Abraham JL, Berry GT, Gracely EJ, Allen RH, Stabler SP. Elevated Methylmalonic Acid and Total Homocysteine Levels Show High Prevalence of Vitamin B12 Deficiency after Gastric Surgery. Ann Intern Med.1996;124:469-76.
Swan HM, Holt DE. Canine gastric adenocarcinoma and leiomyosarcoma: A retrospective study of 21 cases (1986-1999) and literature review. J Am Anim Hosp Assoc 2002; 38:157-164.
Tobias KM. Surgical stapling devices in veterinary medicine: A review. Vet Surg 36 (2007) 341-349.
Walter MC, Matthiesen DT, Stone EA. Pylorectomy and gastroduodenostomy in the dog: Technique and clinical results in 28 cases. JAVMA Nov 1, 1985:187(9):909-14.
Gastric Dilatation-Volvulus
Jacqueline R. Davidson
Acute gastric dilatation with volvulus (GDV) is a medical and surgical emergency, which can be life-threatening. Acute GDV is usually a disease of large and giant breed dogs,1 but can occur in any breed of dog or cat. It typically affects middle-aged or older dogs,1 but there is no sex predilection.
Pathophysiology
Gastric dilatation is generally thought to precede volvulus, although this has not been proven. The composition of entrapped air within the gastric lumen suggests that it is secondary to aerophagia, but may also contain gases produced by fermentation of carbohydrates or diffusion from blood.2 As the stomach continues to dilate, gastric outflow becomes obstructed by compression of the duodenum and esophagus. The stomach rotates around the long axis of the esophagus, with the pylorus moving cranioventrally from right to left (Figure 19-10). The typical location for the pylorus during GDV is dorsal to the esophagus and fundus, on the left aspect of the abdominal cavity. The degree of gastric rotation may range from 0° to 360°, but most are between 180° and 270°. The spleen is attached to the greater curvature, so it is displaced as the stomach rotates and becomes congested. Gastric dilatation-volvulus can cause severe changes in cardiovascular, respiratory, and gastrointestinal physiology.
Gastric dilatation and increased intraabdominal pressure causes obstruction of the portal vein and caudal vena cava, which causes decreased venous return to the heart and portal hypertension with splanchnic vascular pooling. This results in decreased cardiac output with decreased systemic blood pressure and decreased tissue perfusion to major organs. Portal hypertension also causes interstitial edema, which further compromises the microcirculation of the abdominal viscera and contributes to a reduced vascular volume. When there is lack of blood flow to the tissues, waste products and toxins may accumulate in the hypoxic cells. As blood flow is restored, oxygen free radicals may form and cause tissue damage, known as reperfusion injury.

Figure 19-10. A. Normal stomach position when viewed in a ventrodorsal position. B-E. In gastric dilatation-volvulus, the stomach is rotated about the esophagus in a clockwise direction causing malposition of the pylorus, fundus, and spleen. For illustrative purposes, the gastric dilatation has not been pictured. Vessels are included for orientation.
Although cardiac arrhythmias commonly occur with GDV, the etiology is unclear.3 Reduced tissue perfusion stimulates the release of catecholamines, which cause peripheral vasoconstriction and increased heart rate, thereby increasing myocardial oxygen demand. Myocardial ischemia has been implicated as a leading cause of the cardiac arrhythmias.4 Coronary blood flow may be reduced because of the poor venous return and also because of the shortened diastole that occurs with tachycardia. Other factors that could contribute to arrhythmias include substances that are released in association with tissue hypoperfusion or systemic inflammation, electrolyte and acid-base imbalances, and endotoxemia.
Stomach distension prevents normal diaphragmatic excursions, thereby reducing tidal volume. Respiratory rate and effort are increased as a compensatory mechanism, but respiratory acidosis may result. This can exacerbate a metabolic acidosis. Aspiration may occur secondary to vomiting, and this may also impair respiratory function.
Increasing gastric intraluminal pressure impairs perfusion of the gastric wall resulting in hemorrhage, edema, ulceration or necrosis, particularly to the gastric mucosa. More severe pressure will compromise perfusion of the seromuscular layers, and can result in full thickness necrosis with perforation and subsequent peritonitis. Both reduced cardiac output and mechanical kinking or avulsion of vessels can further compromise blood flow to the stomach wall. The short gastric vessels are often affected, but the extensive gastric collateral blood supply makes full-thickness necrosis uncommon.
Local lymphatic tissue that has suffered ischemic damage is unable to prevent translocation of intestinal pathogens from the gastrointestinal mucosa into the circulatory system. Translocation of bacteria to the gut-associated lymphatics is believed to stimulate production of numerous cytokines, which is a factor in promoting the systemic inflammatory response syndrome and multiple organ dysfunction syndrome.6 In addition, impaired return of lymphatic and venous fluid inhibits delivery of pathogens to the immune centers. When circulation is restored, there is potential for release of pathogens and endotoxins into the systemic circulation. Systemic effects include decreased systemic vascular resistance and increased cardiac output, increased vascular permeability, hepatocellular dysfunction, renal tubular damage, microvascular occlusion, and disseminated intravascular coagulation.
Diagnosis
A presumptive diagnosis of GDV or gastric dilatation can often be made based on signalment, history and physical examination. Clinical signs include restlessness, hypersalivation, and unproductive vomiting or retching. A distended, tympanic abdomen is usually obvious, but may not be apparent in some cases. The dog may be dyspneic due to pain, aspiration, or abdominal distention. Dogs may present in compensatory shock, with tachycardia, tachypnea, pale mucous membranes, prolonged capillary refill time, normal pulses, and cold extremities with normothermia. Endotoxic shock differs from compensatory shock in that the membranes may be injected or “muddy”, and fever may be present. Severely affected dogs may be recumbent or comatose. Signs of noncompensatory shock include bradycardia, weak respiration, white or muddy mucous membranes, no capillary refill, poor pulses, cold extremities and hypothermia.
Initial hematology may indicate hemoconcentration (increased packed cell volume and total protein), and a stress or inflammatory leukogram (increased polymorphonuclear leukocytes, increased monocytes, decreased lymphocytes). In cases of decompensation, polymorphonuclear leukocytes are decreased. Thrombocytopenia may be evident on hematology. Changes in activated clotting time, prothrombin time, and partial thromboplastin time may suggest a hypercoagulable state or disseminated intravascular coagulation. Abnormalities of multiple values in the coagulation profile are associated with an increased likelihood of gastric necrosis.7
Initial clinical chemistries may show evidence of liver damage (increased alanine transaminase), biliary stasis (increased total bilirubin), and prerenal or renal azotemia (increased blood urea nitrogen and creatinine). There may also be evidence of blood loss or transudation (lower than expected total protein and albumin) and impaired glucose control. There may be electrolyte abnormalities, particularly hypokalemia. Plasma lactate may be elevated due to anaerobic metabolism or endotoxins.8 Plasma lactate levels are presumably an indication of the degree of systemic hypoperfusion, and higher levels are seen in dogs that are more severely affected clinically.8 Increased lactate concentration may also be associated with gastric necrosis.8 However, change in lactate during the treatment period provides more useful information regarding prognosis than the plasma lactate level at a single point in time.9
Abdominal radiographs are usually not needed to diagnose gastric dilatation, but may be used to confirm the clinical diagnosis or to distinguish between simple gastric dilatation and GDV. Emergency medical therapy is initiated to stabilize the dog before obtaining radiographs. The right lateral recumbent view is the best single view to determine whether the stomach is rotated.10 Radiographs of dogs with gastric dilatation reveal a large, uniform gas-filled gastric shadow and possibly excessive gas in the intestines as well. Gastric dilatation-volvulus is suspected if a tissue density separates the gas-filled gastric shadow into two regions. The gas-filled pylorus may be identified dorsal to the fundus of the stomach. Gas within the gastric wall is suggestive of gastric necrosis, but is not a reliable finding.11 Free gas is present in the abdominal cavity in cases where the stomach has perforated. However, free gas may also be present in those cases where trocarization for gastric deompression was performed prior to obtaining radiographs. Gas in the esophagus may be due to aerophagia and does not necessarily indicate megaesophagus.
Treatment
The initial therapeutic goals are to relieve the gastric distention and treat the cardiovascular compromise. Treatment should begin immediately, and diagnostic tests may be performed as the dog becomes more stable.
Initial Medical Therapy
Aggressive fluid therapy with isotonic crystalloids should be instituted immediately. Several large-bore catheters may need to be placed in order to achieve an adequate rate of fluid administration. The catheters should be in the cephalic or jugular veins, since venous return from the hind limbs may be compromised. The administration rate of crystalloid fluids is 90 ml/kg. A quarter of the total shock dose is administered quickly, and the dog is reassessed. It is preferable to administer crystalloids (10 to 40 ml/kg) in conjunction with high molecular weight fluids (10 to 20 ml/kg hetastarch or 5 ml/kg of 7% hypertonic saline solution in 6% dextran 70 over 5 minutes).12 After initial fluid administration, crystalloid fluids are continued at approximately 20 ml/ kg/hr, depending on the dog’s response to therapy. Dopamine or dobutamine (2 to 5 μug/kg/min) may also be indicated to improve splanchnic blood flow or provide positive inotropic effects. If disseminated intravascular coagulation is suspected, plasma and heparin (100 mg/kg SQ TID) therapy may be instituted.
The acid-base status of dogs with GDV is unpredictable, so specific acid-base therapy should not be administered unless a blood gas analysis has been performed. Acid-base imbalances will generally self-correct as effective circulation is restored. However, if the pH is below 7.2, bicarbonate therapy may be indicated. Oxygen therapy (40 to 100% inspired) administered by face mask, nasal catheter, or oxygen cage may be beneficial to offset the effects of impaired ventilation.
Many dogs develop cardiac arrhythmias in association with GDV.13 The arrhythmias are most commonly ventricular in origin and may include premature ventricular contractions, ventricular tachycardia, or idioventricular tachyarrhythmias. Treatment should be considered if fluid volume has been adequately replaced and the arrhythmia is life threatening or causing poor perfusion. Pain control, and correction of potassium and acid-base abnormalities are important aspects of management prior to specific antiarrythmia therapy. Treatment may be indicated in the presence of R-on-T phenomenon or ventricular tachycardia. Treatment may also be considered if PVCs occur at a rate of more than 20 to 30 per minute, there are runs of PVCs, or PVCs are multifocal in origin. Pulse quality and mucous membrane color should be evaluated and used to guide therapeutic decisions. The initial treatment of choice for ventricular arrhythmias is 2% lidocaine hydrochloride without epinephrine. A slow bolus (1 to 2 mg/kg IV) may be administered until a normal sinus rhythm appears. This may be repeated twice within a 30-minute period if needed, but the entire dose should not exceed 8 mg/kg. If the arrhythmia persists, a continuous intravenous infusion of lidocaine (50 to 100 μug/kg/min) is administered, adjusting the rate based on the dog’s response to therapy. Procainamide (10 to 40 μug/kg/min IV or 6 to 8 mg/kg IM q.i.d.) may be used instead of lidocaine in refractory cases. Antiarrhythmic therapy should continue for three to five days after the arrhythmias resolve.
Treatment with corticosteroids and antibiotics are not necessary in uncomplicated cases of GDV. There is no confirmed clinical benefit from corticosteroids when they are administered after the onset of shock. Risks of corticosteroid use include impaired immune function and possible increased rate of gastrointestinal ulceration. If used, they should be given gradually after adequate volume restoration has been initiated to prevent further hypotension.
If gastric ischemia or necrosis is suspected, broad-spectrum antibiotics such as first- or second- generation cephalosporins or ampicillin are used. Drugs such as deferoxamine and allopurinol have been used experimentally to prevent reperfusion injury, but their use has not been supported by clinical trials.14
During treatment, parameters such as heart rate, pulse character, mucous membrane color, capillary refill time, urine output, plasma oncotic pressure, and blood pressure are monitored to assess cardiac function and tissue perfusion. In addition packed cell volume, total solids, and plasma lactate levels may be useful measurements to monitor response to fluid therapy. Serum cardiac troponin I and cardiac troponin T may be useful markers of myocardial injury.4 Elevated myoglobin can be seen and is not specific to myocardial injury, but may be used as one indicator to help estimate prognosis.5
Gastric Decompression
Gastric decompression should be performed immediately after intravenous catheters have been placed and volume support has been started. Decompression improves venous return, ventilation, and gastric wall perfusion. However, it can also result in systemic release of endotoxins and metabolic waste products that have accumulated in the areas of vascular stasis and ischemia. This may cause deterioration in clinical signs that necessitate further monitoring and treatment. The stomach is decompressed by orogastric intubation or trocarization. Temporary gastrotomy may be used in rare cases. Esophagostomy or nasogastric intubation may be used to provide continued decompression.
Before attempting orogastric intubation, the distance from the dog’s nose to the 13th rib should be measured to estimate the length of tubing needed. A roll of white tape may be placed in the dog’s mouth as a speculum. A well-lubricated stomach tube is passed slowly through the tape roll and into the esophagus and stomach. The tube should be passed gently to avoid esophageal or gastric tears. Rotating the tube as it is advanced may facilitate passage. In some cases, elevation of the forequarters may decrease pressure on the gastroesophageal junction and allow the tube to pass. Failure to pass the tube does not necessarily indicate gastric volvulus, nor does easy passage indicate the presence of a simple dilatation. If the orogastric tube cannot be passed, or if the dog strongly resists the procedure, needle trocarization may be performed first.
To trocarize the stomach, two to four large-bore (14- to 16- gauge) over-the-needle catheters are inserted percutaneously through the abdominal and stomach walls in the region of greatest abdominal distention. The skin should be clipped and aseptically prepared prior to catheter insertion. After some gas has been evacuated from the stomach it may be easier to pass an orogastric tube to remove more gas, fluid and ingesta. Complications, such as peritonitis, are rare with trocarization.
If orogastric intubation is unsuccessful and surgery cannot be performed for an extended period, temporary gastrostomy may be considered to maintain gastric decompression. An inverted “L” line block of 2% lidocaine is performed in the right paracostal region. An incision is made in the anesthetized region. The abdominal musculature is separated between the fibers. The stomach is identified and sutured to the skin edges circumferentially with a continuous suture pattern to provide a good seal. An incision is made in the exposed stomach wall to allow decompression. The temporary gastrostomy has several disadvantages. It does not correct gastric rotation and it increases the risk of peritonitis. In addition it may interfere with the permanent gastropexy and must be repaired prior to performing the definitive abdominal surgery. Therefore, this procedure should only be performed if absolutely necessary.
Client Education
The etiology of GDV is not well understood. It is likely that multiple environmental and hereditary factors play a role.16 Anatomic differences, diet, gastric motility, and gastrointestinal hormones have been studied. Irish setters with an increased thoracic depth to width ratio are at increased risk. Although overeating, pre- or postprandial exercise, large water consumption, and temperament of the dog have been implicated, there is little evidence to support any of these factors as the cause.1,17,18 Factors associated with an increased risk of GDV that were identified in a prospective study of large breed dogs include increasing age, having a first-degree relative with GDV, faster eating speed (for large breed dogs, but not giant breeds), and eating from a raised food bowl.17 Feeding a large volume once daily is associated with an increased risk of GDV compared to feeding a smaller volume twice daily.18 Feeding dry foods containing fats or oils among the first four label ingredients was associated with a significant increased risk of GDV in one retrospective study.19 Poor body condition and a history of chronic health problems are also associated with an increased risk of GDV, although a causal relationship has not been established.18 Therefore, it is recommended that large and giant breed dogs be fed more than once daily using a food bowl that is not elevated. It can also be recommended to avoid breeding dogs that have a first-degree relative that has had GDV. Prophylactic gastropexy may be considered for breeds that have a high risk of developing GDV.20,21
Dogs that have had one episode of GDV are more likely to have another, although gastropexy reduces this likelihood. Clients should be educated regarding the potential for recurrence and the clinical signs that should alert them to seek prompt veterinary care. Survival rates for dogs that are treated surgically for GDV are about 85%.3,13,22-23
References
- Glickman LT, Glickman NW, Schellenberg DB, et al. Incidence of and breed-related risk factors for gastric dilatation-volvulus in dogs. Journal of the American Veterinary Medical Association 2000;216:40-45.
- Caywood DD, Teague HD, Jackson DA, et al. Gastric gas analysis in the canine gastric dilatation-volvulus syndrome. Journal of the American Animal Hospital Association 1977;13:459-462.
- Brourman JD, Schertel ER, Allen DA, et al. Factors associated with perioperative mortality in dogs with surgically managed gastric dilatation-volvulus: 137 cases (1988-1993). Journal of the American Veterinary Medical Association 1996;208:1855-1858.
- Schober KE, Cornand C, Kirbach B, et al. Serum cardiac troponin I and cardiac troponin T concentrations in dogs with gastric dilatation-volvulus. Journal of the American Veterinary Medical Association 2002;221:381-388.
- Adamik KN, Burgener IA, Kovacevic A, et al. Myoglobin as a prognostic indicator for outcome in dogs with gastric dilatation-volvulus. Journal of Veterinary Emergency and Clinical Care 2009; 19:247-253.
- Winkler KP, Greenfield CL, Schaeffer DJ. Bacteremia and bacterial translocation in the naturally occuring canine gastric dilatation-volvulus patient. Journal of the American Animal Hospital Association 2003;39:361-368.
- Millis DL, Hauptman JG, Fulton RB. Abnormal hemostatic profiles and gastric necrosis in canine gastric dilatation-volvulus. Veterinary Surgery 1993;22:93-97.
- de Papp E, Drobatz KJ, Hughes D. Plasma lactate concentration as a predictor of gastric necrosis and survival among dogs with gastric dilatation-volvulus: 102 cases (1995-1998). Journal of the American Veterinary Medical Association 1999;215:49-52.
- Zacher LA, Berg J, Shaw SP, et al. Association between outcome and changes in plasma lactate concentration during presurgical treatment in dogs with gastric dilatation-volvulus: 64 cases (2002-2008). Journal of American Veterinary Medical Association 2010; 236:892-897.
- Hathcock JT. Radiographic view of choice for the diagnosis of gastric volvulus: The right lateral recumbent view. Journal of the American Animal Hospital Association 1984;20:967-969.
- Fischetti AJ, Saunders HM, Drobatz KJ. Pneumatosis in canine gastric dilatation-volvulus syndrome. Veterinary Radiology and Ultrasound 2004;45:205-209.
- Allen DA, Schertel ER, Muir WW, et al. Hypertonic saline/dextran resuscitation of dogs with experimentally induced gastric dilatation-volvulus shock. American Journal of Veterinary Research 1991;52:92-96.
- Brockman DJ, Washabau RJ, Drobatz KJ. Canine gastric dilatation/ volvulus syndrome in a veterinary critical care unit: 295 cases (1986-1992). Journal of the American Veterinary Medical Association 1995;207:460-464.
- Guilford WG, Komtebedde J, Haskins SC, et al. Influence of allopurinol on the pathophysiology of experimental gastric dilatation-volvulus. The Journal of Veterinary Emergency and Critical Care 1995;5:51-60.
- Lantz GC, Bottoms GD, Carlton WW, et al. The effect of 360 gastric volvulus on the blood supply of the nondistended normal dog stomach. Veterinary Surgery 1984;13:189-196.
- Brockman DJ, Holt DE, Washabau RJ. Pathogenesis of acute canine gastric dilatation-volvulus syndrome: Is there a unifying hypothesis? Compendium on Continuing Education 2000;22:1108-1114.
- Glickman LT, Glickman NW, Schellenberg DB, et al. Non-dietary risk factors for gastric dilatation-volvulus in large and giant breed dogs. Journal of the American Veterinary Medical Association 2000;217:1492-1499.
- Raghavan M, Glickman N, McCabe G, et al. Diet-related risk factors for gastric dilatation-volvulus in dogs of high-risk breeds. Journal of the American Animal Hospital Association 2004;40:192-203.
- Raghavan M, Glickman NW, Glickman LT. The effect of ingredients in dry dog foods on the risk of gastric dilatation-volvulus in dogs. Journal of the American Animal Hospital Association 2006; 42:28-36.
- Ward MP, Patronek GJ, Glickman LT. Benefits of prophylactic gastropexy for dogs at risk of gastric dilatation-volvulus. Preventive Veterinary Medicine 2003;60:319-329.
- Rawlings CA, Mahaffey MB, Bement S, et al. Prospective evaluation of laparoscopic-assisted gastropexy in dogs susceptible to gastric dilatation. Journal of the American Veterinary Medical Association 2002;221:1576-1581.
- Glickman LT, Lantz GC, Schellenberg DB, et al. A prospective study of survival and recurrance following the acute gastric dilatation-volvulus syndrome in 136 dogs. Journal of the American Animal Hospital Association 1998;34:253-259.
- Mackenzie G, Barnhart M, Kennedy S, et al. A retrospective study of factors influencing survival following surgery for gastric dilatation-volvulus syndrome in 306 dogs. Journal of the American Animal Hospital Association 2010; 46:97-102.
Gastric Dilatation-Volvulus (GDV): Surgical Treatment
Amelia Simpson
The goals of surgery for a dog with a GDV are to de-rotate the stomach and return other organs to their normal anatomic positions, evaluate the viability of stomach and spleen, perform a splenectomy (partial or complete) and/or a partial gastrectomy if indicated, and perform a perform a permanent gastropexy to prevent recurrence of volvulus. Surgery should take place as soon as the patient is stable enough to undergo anesthesia. If signs of shock are minimal, surgery may be performed immediately after gastric decompression and initiation of fluid therapy. Dogs with moderate or severe signs of shock may be stabilized by gastric decompression and fluid therapy for several hours until the vital signs improve. A prolonged period of stabilization, however, is undesirable, since the blood supply to the rotated stomach may remain compromised even after it has been decompressed.1
Surgical Technique
A ventral midline celiotomy is performed with the incision extending from the xiphiod process of the sternum to a point midway between the umbilicus and pubis. The use of Balfour retractors is recommended to maintain adequate exposure of the abdominal cavity. The surgeon should evacuate free blood form the abdomen and any actively bleeding short gastric vessels should be ligated. Most commonly, with the dog in dorsal recumbency, the stomach rotates 180° to 270° clockwise around the long axis of the esophagus. In this position, the ventral leaf of the omentum covers the ventral aspect of the displaced stomach which is readily apparent to the surgeon after the abdomen is opened (See Figure 19-10A-E). A clockwise rotation causes the pylorus and the gastric antrum to become displaced from the right ventral body wall and move ventrally over the gastric fundus and body to become positioned adjacent to the esophagus along the left body wall (See Figure 19-10D and E). The maximum rotation of the stomach in the clockwise rotation is 360°. Counterclockwise rotation, although uncommon, has a maximum rotation of 90°. When counterclockwise rotation occurs, the pylorus and antrum move dorsally along the right body wall to a position adjacent to the esophagus. Counterclockwise rotation direction, causes minimal ventral displacement of the gastric fundus and body and the omentum does not cover the ventral aspect of the displaced stomach.2 Depending upon the degree of gastric rotation, the spleen can be found in various positions within the cranial abdomen. Occasionally the spleen may undergo torsion around its vascular pedicle.
To de-rotate the stomach for a 180° clockwise rotation, the fundus, which is located near the right abdominal wall, is depressed in a dorsal direction and the pylorus, abnormally located on the dorsal left, abdominal wall is retracted ventrally and from the left to right side of the abdomen. To prevent further trauma to the gastrosplenic vessels, the spleen is placed in its normal position as gastric de-rotation is performed. If significant distension is present, the stomach may be impossible to de-rotate. In this case, gastric decompression is necessary and can be achieved by passage of an orogastric tube, trocharization, or by a combination of these two methods. If an orogastric tube cannot be advanced into the stomach, trocharization of the stomach should be performed. A large bore needle or IV catheter is placed into the gastric lumen through an area of the stomach wall that appears. The needle/catheter can be connected to suction for rapid removal of accumulated gas and fluid and usually, the orogastric tube can then be successfully passed into the stomach. Following successful decompression, the needle/catheter is removed and the stomach is de-rotated as described above. Suturing of the trocharization site is not necessary, unless a tear was created in the gastric wall during needle placement. The surgeon should be certain that the stomach is completely de-rotated and in a normal anatomic location. This can be achieved by visualizing the junction of the intra-abdominal esophagus and cardia of the stomach and noting a lack of tissue folds in the area. After confirming complete de-rotation of the stomach, a complete abdominal exploration is performed.
During the abdominal exploration, particular attention is paid to the viability of the gastric wall and spleen. Initially, the spleen is often enlarged and congested and its viability may appear questionable. Often, after return to its normal anatomic rotation the spleen begins to return to its normal size and color and splenectomy is not required. Thrombosis of the splenic artery and/or vein can be detected by careful palpation and, if identified, requires partial or complete splenectomy. If the spleen has undergone torsion around its vascular pedicle, it should be removed. In order to prevent deleterious toxin release, splenectomy is performed without de-torsing the splenic vasculature. Splenectomy is required in approximately 25% of cases of GDV.
Partial Gastrectomy
Approximately 10% of dogs with GDV have necrosis along the greater curvature of the fundus or body of the stomach.3 Perforation early in the course of disease due to gastric necrosis is rare. Several methods have been described to evaluate viability of the gastric wall. These include: clinical assessment of serosal color and gastric wall texture, the use of intravenous fluroesceine dye, and nuclear scintigraphy. In a study evaluating the use of fluoresceine fluorescence to predict gastric viability, it was found that fluoresceine fluorescence was only 58% accurate.4 Nuclear scintigraphy, while accurate in assessing stomach wall viability, is impractical in a clinical setting.5-7 Gastric viability determined by clinical assessment of serosal color and gastric wall texture was accurate in 85% of cases of dogs with GDV in one study.4 Since clinical assessment of gastric tissue viability is not always accurate, it is possible to remove tissue which may survive as well as to leave tissue behind which may subsequently necrose and lead to gastric perforation hours to days later. General guidelines for partial gastric resection include in Table 19-2.

Ischemic or necrotic gastric tissue can be excised by partial gastrectomy, or it can be invaginated by an inverting or pursestring suture technique and left to be auto-digested within the gastric lumen. I prefer to perform partial gastrectomy and gastric wall closure with staples or suture (Figure 19-11A-C). The stomach is packed off with moistened laparotomy pads to prevent contamination of the abdomen from spillage of gastric contents. Doyen forceps or stay sutures are placed in viable gastric tissue and can be used to help prevent leakage of gastric contents. The necrotic portion of the stomach wall is resected with a scalpel to the point where there is active arterial bleeding at the surgical margin. A monofilament, absorbable or non-absorbable, suture material is used for a two layer closure. A full thickness simple continuous suture pattern followed by an inverting pattern such as a Cushing’s oversew is recommended. Alternatively, a thoraco-abdominal stapler can be used followed by a Cushing’s oversew using a monofilament, absorbable or non-absorbable suture material. Mortality rates are known to be higher in animals where gastric wall excision is required. The owners should be given a poor prognosis in cases where there is complete gastric necrosis or necrosis of the cardia and abdominal esophagus.

Figure 19-11. Two variations of partial gastrectomy are shown. In A-C, stay sutures are placed to elevate the stomach and to minimize leakage. Necrotic tissue is excised with a rim of viable tissue (dotted line). A two-layer inverting closure is used. In D-l, atraumatic clamps are placed across viable tissue, and the necrotic tissue is excised. The stomach body is subsequently closed with a Parker-Kerr suture line. The first inverting layer of suture is placed over the clamps. The clamps are subsequently removed as the suture line is pulled tight to invertthe suture line. A second inverting suture line completes the closure. (Redrawn in part from Matthiesen DT. Gastric dilatation-volvulus syndrome. In: Slatter DH, ed. Textbook of small animal surgery. Philadelphia: WB Saunders, 1995:580-593.)
Gastropexy
Mortality rates for dogs with a GDV have been reported to be as high as 23%.8 Studies have shown that dogs who receive an effective permanent gastropexy at the time of surgery have less than a 5% chance of recurrence of GDV, whereas dogs who do not receive a gastropexy during surgery have a 54.5-80% rate of recurrence within the first year.8,9 Based on these results, it is strongly recommended that all dogs with a GDV should have a permanent gastropexy performed during surgery for gastric repositioning. Several effective gastropexy techniques have been described including: tube gastropexy, incisional gastropexy, circumcostal gastropexy, belt-loop gastropexy, fundic gastropexy and laparoscopic gastropexy.10-14 Gastrocolopexy and gastrojejunostomy have also been described, but are rarely performed in a clinical setting and are no longer recommended.15,16
I prefer to perform an incisional gastropuexy for prevention of GDV. It is simple to perform, can be completed quickly, has minimal complications, and creates a strong adhesion between the pyloric antrum and the right abdominal wall. The gastropexy is performed only after confirming that the stomach is fully de-rotated and in normal anatomic rotation. The procedure is easier to perform with the surgeon standing on the left side of the dog. A 5-6 cm partial thickness incision is made in the serosal and muscularis layers of the pyloric antrum, midway between the greater and lesser curvature of the stomach, along the long axis of the stomach (Figure 19-12). A corresponding incision is made in the right lateral body wall just caudal to the last rib. The body wall incision should extend through the peritoneum and transverse abdominal muscle. The gastric incision edges are sutured to the corresponding body wall incision edges using a monofilament absorbable (polydioxanone) or non-absorbable suture in a simple continuous pattern (Figure 19-13). The suture should not enter the gastric lumen as this may lead to fistula formation and/or sepsis. Routine abdominal closure is performed after copious lavage.

Figure 19-12. The location of the gastropexy site in the area of the pyloric antrum.
Post-Operative Care and Complications
Intensive nursing care should be provided during the postoperative period. Pain control, maintenance of vascular volume, and return to normal alimentation and gastric motility are important. Pain is controlled with injectable hydromorphone (0.05-0.1 mg/ kg, SC or IV q4-6hrs) or injectable burenorphine (0.02 mg/kg, SC or IV, q6hrs) for 48-72 hours after surgery. Since these dogs usually have extended hospitaliztion times, they are comfortable by the time of discharge and analgesic medication is not routinely necessary after discharge. If the dog appears painful, Tramadol (1-4 mg/kg, PO, q8hrs) can be prescribed to be administered at home for 3-5 days. Dogs are routinely maintained on intravenous fluids for the first 24-36 hours postoperatively for maintenance and continuing fluid losses, or until they are eating and drinking without vomiting. Food and water are withheld for the first 18-24 hours postoperatively unless the animal is hypoalbuminemic. Ice chips or small quantities of water are offered in small amounts. If there is no vomiting after several hours of starting the patient on water, food is offered in small amounts every 4-6 hours and the patient is gradually returned to normal alimentation over 24-48 hours.
If vomiting occurs, potassium levels should be assessed as hypokalemia is common and may promote ileus. Prokinetic drugs (metoclopramide or cisapride, ranitidine, erythromycin) and gastric acid-inhibitors (cimetidine, ranitidine, famotidine, omeprazole) are beneficial in animals that have vomiting or regurgitation postoperatively.
Antibiotics administered perioperatively are continued in animals that have abdominal contamination during surgery secondary to gastric necrosis and in animals that are suspected of having endotoxic shock initially.
Cardiac Arrhythmias
After surgery, dogs are observed for cardiac arrhythmias with continuous ECG monitoring for 48-72 hours. The most common arrhythmia seen in these patients is ventricular premature contractions (VPCs) although supraventricular arrhythmias such as atrial fibrillation and atrial premature depolarization have also been observed.17,18 Electrolyte levels should be checked and corrected if abnormal prior to considering therapy for arrythmias. Patients with VPCs occurring at a high rate (200-240+ beats per minute) who appear clinically affected by the arrhythmia (weak pulse quality, poor mucous membrane color, prolonged capillary refill time, and weakness/lethargy) should be treated with antiarrhythmic medication. Most commonly, lidocaine hydrochloride at a dose of 2-4 mg/kg, is administered as an intravenous bolus given slowly to effect. The lidocaine dose can be repeated and, if necessary, it can be continued at a constant rate infusion of 0.05-0.08 mg/kg/min. If the arrhythmia is refractory to lidocaine, procainamide can be used. Procainamide should be administered at a dose of 6mg/kg as an IV bolus over 5 minutes, followed by a 10 to 25 ug/kg/min constant rate infusion. The dog should be placed on oral procainamide at a dose of 10 mg/kg every 8 hours for 15 days. Long-term antiarrhythmic treatment is usually unnecessary as the arrhythmias usually resolve.
Sepsis (Gastric Necrosis/Perforation)
The risk of dehiscence following partial gastrectomy is greatest within the first 72 hours after surgery. Dogs are monitored closely for signs of peritonitis such as lethargy, abdominal pain and/or distension, vomiting, hypotension, hypoglycemia, and hyper- or hypothermia. If dehiscence is suspected, a CBC and abdominocentesis should be performed. The abdominal fluid should be evaluated for the presence of intracellular bacteria. A paired sample of the abdominal fluid and blood should be measured for glucose levels. A glucose level in the abdominal effusion that is more than 20 mg/dl lower than the blood glucose is indicative of septic peritonitis and warrants re-exploration.19
Prophylactic Gastropexy
Prophylactic gastropexy is currently recommended for dogs who have had a spontaneous episode of gastric dilatation, in dogs that have a first-degree relative who has had GDV, and in some breeds that are at high risk for the development of GDV such as Great Danes, Irish Setters, and Bloodhounds. Prophylactic gastropexy can be performed at the time of elective neutering in susceptible breeds. Any of the previously mentioned gastropexy techniques can be performed by an open approach. Rawlings and colleagues have performed laparoscopic-assisted prophylactic gastropexy in 23 dogs susceptible to GDV and found that it resulted in a persistent attachment between the stomach and abdominal wall, few complications and no occurrence of GDV within one year of the original surgery.20 Clinically, laparoscopic assisted gastropexy has proven an easy and effective prophylactic gastropexy technique.20
References
- Lanz GC, Bottoms GD, Carlton WW, et al. The effect of 360 gastric volvulus on the blood supply of the nondistended normal dog stomach. Vet Surg 1984; 13:189-196.
- Brockman DJ, Holt DE, Washabau RJ. Pathogenesis of acute gastric dilatation-volvulus syndrome: Is there a unifying hypothesis? Compend Cont Educ Pract Vet 2000;22:1108-1114.
- Matthiesen DT: Partial gastrectomy as treatment of gastric volvulus: Results in 30 dogs. Vet Surg 14: 185-193, 1985.
- Wheaton LG, Thacker HL, Caldwall S: Intravenous fluroescein as an indicator of gastric viability in gastric dilation-volvulus. J Am Anim Hosp Assoc 22:197-204, 1986.
- Berardi C, Twardock AR, Wheaton LG, et al. Nuclear imaging of the stomach of healthy dogs. Am J Vet Res 1991;52:1081-8
- Berardi C, Wheaton L, Twardock AR, et al. Nuclear imaging to evaluate gastric mucosal viability following surgical correction of gastric dilatation/volvulus. J Am Anim Hosp Assoc 1993;29:239-46.
- Berardi C, Wheaton L, Twardock AR, et al. Use of nuclear imaging technique to detect gastric wall ischemia. Am J Vet Res 1991;52:1089-96.
- Glickman LT, Glickman NW, Schellenberg DB, et al. Non-dietary risk factors for gastric dilatation-volvulus in large and giant breed dogs. J Amer Vet Med Assoc 2000;217:1492-1499.
- Wingfield WE, Betts CW, Greene RW. Operative techniques and recurrence rates associated with gastric volvulus in the dog. J Sm Anim Pract 1975;16:427-32.
- Parks JL, Green RW: Tube gastrostomy for the treatment of gastric volvulus. J Am Anim Hosp Assoc 12:168-172, 1976.
- MacCoy DM, Sykes GP, Hoffer RE, et al: A gastropexy technique for permanent fixation of the pyloric antrum. J Am Anim Hosp Assoc 18:763- 768, 1982.
- Fallah AM, Lumb WV, Nelson AW, et al: Circumcostal gastropexy in the dog: A preliminary study. Vet Surg 11;9-12, 1982.
- Whitney WO, Scavelli TD, Mattheisen DT, et al: Belt-loop gastropexy: Technique and surgical results in 20 dogs. J Am Anim Hosp Assoc 25:75-83m 1989.
- Myer-Lindenberg A, Harder A, Fehr M, et al: Treatment of gastric dilatation-volvulus and a rapid method for prevention of relapse in dogs: 134 cases. J Am Vet Med Assoc 203:1303-1307, 1993.
- Christie TR, Smith CW: Gastrocolopexy for prevention of recurrent gastric volvulus. J Am Anim Hosp Assoc 12:173-176, 1976.
- Pritchard D: Prevention of acute gastric dilation by gastrojejunostomy. Canine Pract 4:51-55, 1977.
- Muir WW, Lipowitz AJ: Cardiac dysrhythmias associated with gastric dilatation-volvulus in the dog. Am J Vet Res 172:683, 1978.
- Muir WW, Bonagura JD: Treatment of cardiac arrhythmias in dogs with gastric distension-volvulus. J Am Vet Med Assoc 184:1366, 1984.
- Bonczynski JJ, Ludwig LL, Barton LJ, et al: Comparison of peritoneal fluid and peripheral blood pH, bicarbonate, glucose, and lactate concentration as a diagnostic tool for septic peritonitis in dogs and cats. Vet Surg 32:161-166,2003.
- Rawlings CA, Mahaffey MB, Bement S, et al: Prospective evaluation of laparoscopic-assisted gastropexy in dogs susceptible to gastric dilation. J Am Vet Med Assoc 221:1576-1581.
Incisional Gastropexy
Douglas M. MacCoy
Gastric volvulus is a serious, often fatal problem that occurs primarily in large, deep-chested dogs. Gastropexy,1,2 gastroplasty,3 tube gastrostomy,4 and gastrocolopexy5 have all been used in an attempt to fix the stomach to the body wall permanently and to prevent recurrent volvulus. The incisional gastropexy1 offers a method of producing a permanent gastropexy without the potential complications and aftercare associated with tube gastrostomy. It may be used as an alternative to a tube gastrostomy when postoperative decompression will be provided by pharyngostomy tube or is not thought necessary, but a permanent gastropexy is still desired.6 The same low potential for complications also makes it suitable as a prophylactic procedure in high-risk patients.
Surgical Technique
The cranial abdomen is approached by a ventral midline laparotomy. The pyloric antrum is identified and is held in the surgical field by thumb forceps, Babcock forceps, or stay sutures. Using a scalpel, the surgeon makes an incision equal in length to the diameter of the duodenum through the gastric serosa and into but not through the muscularis over the parietal surface of the pyloric antrum equidistant from the attachments of the greater and lesser omenta (See Figure 19-13A). The incision should be at least one duodenal diameter away from the pylorus, to avoid distortion of the pylorus. A second incision of the same length is made through the peritoneum and internal fascia of the rectus abdominis muscle or transversus abdominis muscle of the ventrolateral abdominal wall adjacent to the incision on the pyloric antrum (See Figure 19-13B).
The edges of the abdominal wall incision are sutured to the edges of the antral incision using 2-0 or 1-0 monofilament nylon or polypropylene in a simple continuous pattern, creating an imperforate, circular stoma (See Figure 19-13C). The abdominal incision is closed in a routine fashion.

Figure 19-13. A. Initial pyloric antrum incision. B. Matching incision on body wall. C. Suturing of body wall and pyloric antrum.
Postoperative Care
Exercise is restricted for a minimum of 3 weeks to allow healing of the abdominal incision. No dietary restrictions are needed.
Editor’s Note: Techniques such as gastrocolopexy do not prevent gastric volvulus. I pefer the incisional gastropexy technique described however I make the antral incision 5-6 cm in length. Many surgeons prefer a long-acting absorbable suture such as polydioxanone for gastropexy.
References
- MacCoy DM, Sykes GP, Hoffer RE, et al. A gastropexy technique for permanent fixation of the pyloric antrum. J Am Anim Hosp Assoc 1982;18:763-768.
- Woolfson JM, Kostolich M. Circumcostal gastropexy: clinical use of the technique in 34 dogs with gastric dilation-volvulus. J Am Anim Hosp Assoc 1986;22:825-830.
- Matthiesen DT. Partial gastrectomy as treatment of gastric volvulus; results in 30 dogs. Vet Surg 1985;14:185-193.
- Parks JL, Greene RW. Tube gastrostomy for the treatment of gastric volvulus. J Am Anim Hosp Assoc 1976;12:168-172.
- Christie TR, Smith CW. Gastrocolopexy for prevention of recurrent gastric volvulus. J Am Anim Hosp Assoc 1976;12:173-176.
- Lindgren WG, Mullen HS, Marino DJ, et al. Long-term follow-up and clinical results of incisional gastropexy for repair of gastric dilation-volvulus syndrome. In: Proceedings of the Fifth American College of Veterinary Surgeons Veterinary Symposium. American College of Veterinary Surgeons. Chicago, IL. 1995:11.
Circumcostal Gastropexy
Gary W. Ellison
The circumcostal gastropexy technique1 has become popular with many small animal surgeons because it forms a stronger adhesion than the tube gastrostomy or incisional gastropexy technique.2 Other potential advantages of this technique include a viable muscle flap adhesion and a more proper anatomic placement of the stomach.1 Potential disadvantages of the circumcostal gastropexy technique include possible rib fracture or creation of pneumothorax when the operation is performed by surgeons who are inexperienced with the technique.3 This procedure is also reported to be more technically demanding and time-consuming to perform than other gastropexy techniques, but I disagree with this statement.
Surgical Technique
To perform the circumcostal gastropexy, two 1x4 cm partial-thickness gastric flaps are created and are wrapped around either the eleventh or twelfth costal cartilage. Initially, 2-0 polypropylene stay sutures are placed 2 and 8 cm proximal to the pylorus, respectively. A transverse nick incision is then made 3 cm distal to the pylorus with Metzenbaum scissors. A second transverse nick incision is made at the other end 4 cm proximal to the first. To avoid penetrating the gastric lumen when making these incisions, the serosa and muscularis are first grasped between the surgeon’s thumb and forefinger. This maneuver separates these layers from the underlying submucosa. The two transverse incisions are then connected with a scalpel or scissors. The seromuscular layer is then separated from the submucosa with scissors on both sides, thereby creating two 1.0x4 cm seromuscular pedicle flaps (Figure 19-14). The chondral portion of the right eleventh or twelfth rib is encircled with towel clamps from the abdominal surface, and the peritoneum and transverse abdominal muscle are incised with a No. 10 blade (Figure 19-15). A tunnel is then made around the medial aspect of the rib using Metzenbaum scissors. If the eleventh or twelfth rib is used, the incision is caudal to all diaphragmatic attachments, and the thoracic cavity is not entered. The caudal arm of each stay suture is then passed through the rib tunnel, and the stomach is pulled up against the right abdominal wall. The caudal muscular flap is passed around the lateral aspect of the rib with a stay suture or grasping forces, and the two stay sutures are tied around the rib (Figure 19-16). The caudal muscular flap is then apposed to the cranial muscular flap with simple interrupted sutures of 2-0 polypropylene (Figure 19-17). A second layer of simple interrupted sutures of 3-0 po lypropylene is used to appose gastric serosa to the incised transversus muscle (Figure 19-18). The ventral midline is then closed routinely. Postoperative care is similar to that for other gastropexy techniques.
Results
In one study, 30 patients with GDV were followed-up for an average of 12.7 months after circumcostal gastropexy with contrast studies or necropsy.4 Of the animals that survived, just 1 dog (3.3%) suffered a clinical recurrence of gastric dilatation. In another study, 34 dogs were followed-up after circumcostal gastropexy, with a mean follow-up of 11.3 months. One patient developed peritonitis as a result of inadvertent penetration of the gastric wall during flap formation. The overall mortality was 8.8%, and suspected recurrence occurred in 2 patients (6.9%) because of a second episode of gastric distension.5 Neither of these recurrences was documented with radiographs or necropsy as due to gastropexy failure.

Figure 19-14. After placing stay sutures, two small incisions are made through the seromuscular layer of the pyloric antrum and are connected with scissors. The flaps are undermined for a distance of I cm. (From Ellison GW. Gastric dilatation volvulus: surgical prevention. Vet Clin North Am Small Anim Pract 1993:23:524.)
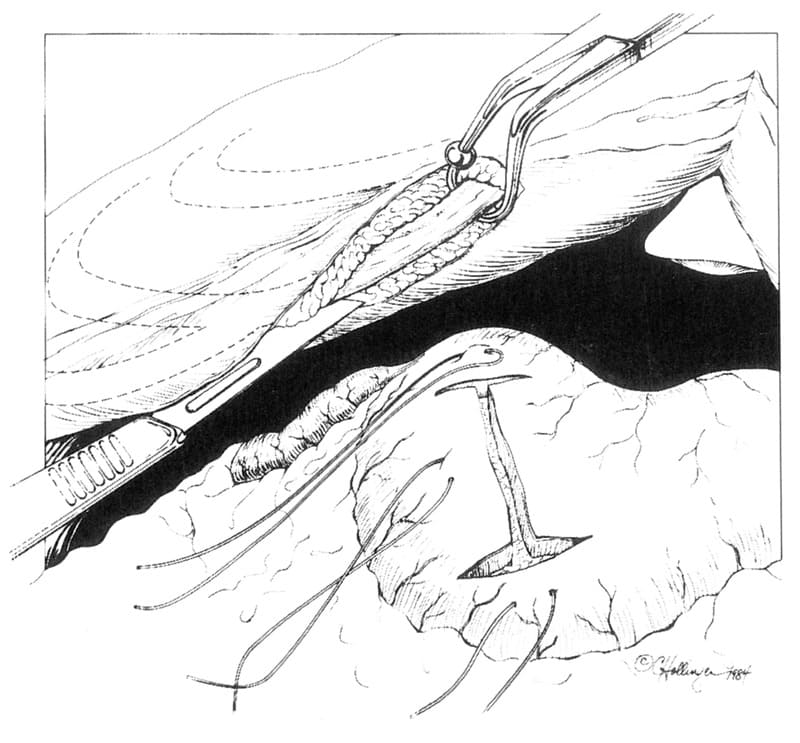
Figure 19-15. The eleventh or twelfth rib is then grasped with towel clamps from the abdominal surface, and the peritoneum and transverse abdominal muscle are incised with a No. 10 blade. (From Ellison GW. Gastric dilatation volvulus: surgical prevention. Vet Clin North Am Small Anim Pract 1993:23:524.)

Figure 19-16. After dissecting lateral to the rib, the caudal flap is brought around, and the two stay sutures are tied. (From Ellison GW. Gastric dilatation volvulus: surgical prevention. Vet Clin North Am Small Anim Pract 1993:23:524.)
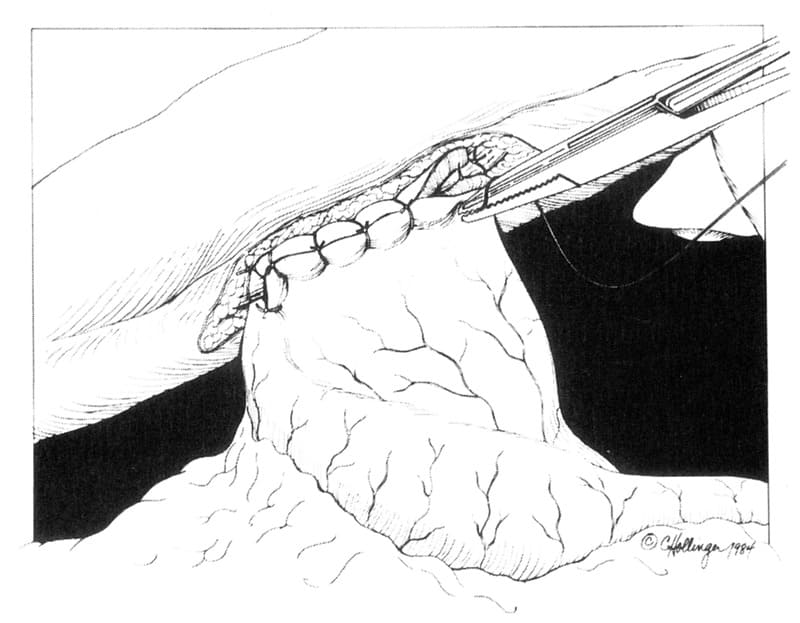
Figure 19-17. The two flaps are then apposed with simple interrupted sutures. (From Ellison GW. Gastric dilatation volvulus: surgical prevention. Vet Clin North Am Small Anim Pract 1993:23:524.)

Figure 19-18. A second layer of simple interrupted sutures apposes the stomach wall with the incised transverse abdominal muscle and completes the procedure. (From Ellison GW. Gastric dilatation volvulus: surgical prevention. Vet Clin North Am Small Anim Pract 1993:23:524.)
References
- Fallah AM, Lumb WV, Nelson AW, et al. Circumcostal gastropexy in the dog: a preliminary study. Vet Surg 1982;11:19-22.
- Fox SM, Ellison GW, Miller GJ. Observations on the mechanical failure of three gastropexy techniques. J Am Anim Hosp Assoc 1985;21:739-734.
- Leib MS, Blass CE. Gastric dilatation-volvulus in dogs: an update. Compend Contin Educ Pract Vet 1984;6:961-967.
- Leib MS, Konde LJ, Wingfield WE, et al. Circumcostal gastropexy for preventing recurrence of gastric dilatation-volvulus in the dog: an evaluation of 30 cases. J Am Vet Med Assoc 1985; 187:245-248.
- Woolfson JM, Kostolich M. Circumcostal gastropexy: clinical use of the tech nique in 34 dogs with gastric dilatation-volvulus. J Am Anim Hosp Assoc 1986;22:825-830.
Laparoscopic Assisted Gastropexy
Don R. Waldron
Introduction
Creation of a permanent gastropexy is the single most important factor in preventing gastric-dilatation volvulus (GDV) in susceptible dogs. Gastropexy is performed most commonly as part of therapeutic surgery following gastric repositioning for animals with gastric volvulus. Performance of an effective gastropexy includes fixation of the gastric antrum to the right abdominal wall or rib area. Many methods of gastropexy have been described and are effective if performed well technically. Incisional gastropexy is a simple and effective technique that has been widely used and is simple to perform.
Increased knowledge of GDV among veterinarians and owners and identification of known risk factors for dogs susceptible to the condition has resulted in increased numbers of dogs having prophylactic gastropexy to prevent gastric volvulus. Risk factors for development of the disease include first-degree relatives who have been affected (genetic), stress, breed/confirmation (Great Danes, St, Bernards, Weimeraners, Irish Setters, Gordon Setters, Standard Poodles) and diet.1 It is widely accepted that animals with deep thorax-to-width ratios are especially at risk for the disease. Because of high mortality rates associated with GDV, prophylactic gastropexy should be considered in dogs identified as being at high risk.
Prophylactic gastropexy can be performed by open laparotomy concurrently with elective ovariohysterectomy in the female however the incision required is a substantial one extending from xiphoid caudal to the umbilicus. Laparoscopic gastropexies have been described however a high degree of laparoscopic surgical skill and experience are required.2 The laparoscopic-assisted gastropexy technique described here was developed and described by Rawlings et al in 2001 and 2002.3,4 The technique was investigated in the laboratory and has been widely used on client owned dogs with minimal morbidity and a high degree of success in preventing GDV. Two portals are used and the laparoscope is used to identify and grasp the antrum, however, once the antrum is exteriorized the gastropexy is performed as an open technique. Minimal laparoscopic skills are required making the technique relatively easy to perform.

Figure 19-19. The dog is placed in dorsal recumbency and two abdominal incisions are made, midline for the laparoscope and right side caudal to the last rib for the gastropexy.
Figure 19-20. The antrum is grasped with tissue forceps midway between the greater and lesser curvatures about 5 to 7 cm orally from the pylorus. The forceps, the right-sided trocar cannula and the antrum are exteriorized through the lengthened cannula incision.
Technique
General anesthesia is induced and positive-pressure ventilation used during laparoscopic surgery. The dog is placed in dorsal recumbency and the complete abdomen prepared for surgery. A trocar cannula is placed 2cm caudal to the umbilicus which accommodates the laparoscope (Figure 19-19). The peritoneal cavity is distended with carbon dioxide and a 0°, 5°, or 10° laparoscope connected to a light source and video camera is inserted through the cannula. A second trocar cannula is placed 3 cm caudal to the last rib and lateral to the rectus abdominis muscle (See Figure 19-18). A 10mm Babcock forcep is placed into the abdomen to grasp the gastric antrum midway between the greater and lesser curvatures approximately 5 to 7 cm proximal to the pylorus. Allis tissue forceps are more traumatic but are useful in grasping the antrum if the stomach slips from the Babcock forceps.
The tissue forceps and antrum are exteriorized by removing the right side cannula and extending the cannula incision to 4cm in length in a direction parallel to the last rib. Care is taken to assure the antrum is not twisted as it is withdrawn from the abdomen (Figure 19-20). Size 0 “stay” sutures are placed 4 to 5 cm apart through the gastric serosa/muscularis to control the gastric wall during gastropexy. A seromuscular longitudinal incision is made in the gastric wall between the stay sutures. The sides of the gastric wall incision are dissected free from the mucosa so that adequate gastric tissue is available for the gastropexy and so that sutures are not placed through the gastric mucosa. A simple continuous pattern of size 0 or 2-0 synthetic monofilament absorbable suture (polydioxanone) is used to appose the seromuscular layer of the stomach to the transversus abdominus muscle (Figure 19-21). A separate suture line is placed on the cranial and caudal aspects of the gastropexy site. The abdominal oblique muscles are closed with interrupted absorbable sutures and the remainder of the incision by surgeon’s choice. The completed gastropexy site is viewed intraabdominally (Figure 19-22) to assure that the gastric antrum is not twisted, the midline cannula is removed and the incision is closed.

Figure 19-21. A seromuscular incision is made in the gastric wall down to the gastric mucosa and the sides of the incision dissected in both directions from the primary incision. The seromuscular layer is sutured to the transversus abdominis muscle with a continuous pattern of monofilament absorbable suture. SM=seromuscular layer of the stomach; GM=gastric mucosa; TA= transversus abdominis muscle.

Figure 19-22. Laparoscopic view of the completed gastropexy with no evidence of antral twisting.
References
- Glickman LT, Glickman NW, Perez CM, et al. Analysis of risk factors for gastric dilatation-volvulus in dogs. J Amer Vet Med Assoc 1994; 204: 1465-1471.
- Hardie RJ, Flanders JA, Schmidt P, et al. Biomechanical and histological evaluation of a laparoscopic stapled gastropexy technique in dogs. Vet Surg 1996;25: 127-133.
- Rawlings CA, Foutz TL, Mahaffey MB, et al. A rapid and strong laparoscopic-assisted gastropexy in dogs. Am J Vet Res 2001; 62: 871-875.
- Rawlings CA. Laparoscopic-assisted gastropexy. J Am Anim Hosp Assoc 2002; 38: 15-19.
Get access to all handy features included in the IVIS website
- Get unlimited access to books, proceedings and journals.
- Get access to a global catalogue of meetings, on-site and online courses, webinars and educational videos.
- Bookmark your favorite articles in My Library for future reading.
- Save future meetings and courses in My Calendar and My e-Learning.
- Ask authors questions and read what others have to say.
About
How to reference this publication (Harvard system)?
Author(s)
Copyright Statement
© All text and images in this publication are copyright protected and cannot be reproduced or copied in any way.Related Content
Readers also viewed these publications
Buy this book
Buy this book
This book and many other titles are available from Teton Newmedia, your premier source for Veterinary Medicine books. To better serve you, the Teton NewMedia titles are now also available through CRC Press. Teton NewMedia is committed to providing alternative, interactive content including print, CD-ROM, web-based applications and eBooks.
Teton NewMedia
PO Box 4833
Jackson, WY 83001
307.734.0441
Email: [email protected]







Comments (0)
Ask the author
0 comments