

Get access to all handy features included in the IVIS website
- Get unlimited access to books, proceedings and journals.
- Get access to a global catalogue of meetings, on-site and online courses, webinars and educational videos.
- Bookmark your favorite articles in My Library for future reading.
- Save future meetings and courses in My Calendar and My e-Learning.
- Ask authors questions and read what others have to say.
Intestines
Get access to all handy features included in the IVIS website
- Get unlimited access to books, proceedings and journals.
- Get access to a global catalogue of meetings, on-site and online courses, webinars and educational videos.
- Bookmark your favorite articles in My Library for future reading.
- Save future meetings and courses in My Calendar and My e-Learning.
- Ask authors questions and read what others have to say.
Read
Enterotomy
Gary W. Ellison
Indications
The most common indication for enterotomy in small animals is to remove intraluminal intestinal foreign bodies that cause obstruction. Foreign bodies can be present in animals of any age, but they are most common in puppies or kittens because of indiscriminate eating habits. Common intestinal foreign bodies in dogs include bones, balls, corncobs, and cellophane wrappers. Cats commonly ingest sharp foreign bodies (e.g., straight pins and needles) and linear foreign bodies (e.g., yarn, tinsel, fishing line, and string meat wrappings). Enterotomy also is performed as a biopsy technique and to examine the intestinal lumen for evidence of mucosal ulceration, strictures, or neoplasia. Superficial ulcerations or intestinal polyps sometimes can be resected via enterotomy, but most intramural lesions require intestinal resection and anastomosis.
Pathophysiology and Preoperative treatment of Intestinal Obstruction
Animals with incomplete intestinal obstruction caused by intraluminal foreign bodies or neoplasia usually vomit sporadically or are anorectic. Surprisingly, sharp foreign bodies such as nails, straight pins, and bones often pass spontaneously through the entire gastrointestinal tract without causing a perforation. Conversely, complete intraluminal obstructions usually cause acute bowel distension and unrelenting clinical signs. With proximal (duodenal) obstructions, vomiting may be projectile. With distal jejunal or ileal obstructions, vomiting may be seen early in the course of the disease, but anorexia and bowel distension follow. After obstruction of the midjejunum in dogs, vomiting often decreases to once a day after 24 to 36 hours, and many dogs can live for several weeks if hydration is maintained.
Most intestinal obstructions are distal to the bile and pancreatic ducts, resulting in loss of highly alkaline duodenal, pancreatic, and biliary secretions. Metabolic acidosis usually occurs from loss of these bicarbonate-rich duodenal contents. Dehydration should be corrected and maintenance fluid needs are usually met with a balanced electrolyte solution such as lactated Ringer’s solution. Potassium chloride supplementation of fluids may be indicated, depending on the patient’s acid-base status and serum potassium level. With obstructions at the pylorus or proximal duodenum, gastric fluids rich in potassium, sodium, hydrogen ion, and chloride are vomited, and metabolic alkalosis with hypochloremia, hyponatremia, and hypokalemia may result. In those cases, dehydration is corrected with intravenous 0.9% sodium chloride solution supplemented with potassium chloride depending on the patient’s preoperative serum potassium level.
Surgical Technique
A ventral midline laparotomy incision is made from the xiphoid to the pubis. The entire intestinal tract should be evaluated to determine the number of foreign bodies and assess the viability of the bowel wall. The affected bowel segment is isolated from the remainder of the viscera with saline-soaked laparotomy sponges. In patients with a complete obstruction, intestinal distension proximal to the obstruction is often profound, and the distended loops of bowel usually take on a congested or cyanotic appearance (Figure 20-1A).
Intestinal viability is best evaluated after decompression of fluid and gas from dilated loops of intestine. Decompression is performed with a 20-gauge needle and suction apparatus or a 60-mL syringe with a three-way stopcock. If intestinal wall ischemia and necrosis are present, resection and anastomosis must be performed (following article). In most cases of simple mechanical obstruction, however, bowel viability is maintained, and the gross appearance of dark, distended loops of bowel improves rapidly after decompression and removal of the obstruction.

Figure 20-1. A. Complete intestinal obstruction caused by a luminal foreign body such as a corncob causes fluid and gaseous distension. Congestion or cyanotic appearance of the bowel wall occurs proximal to the obstruction. B. An enterotomy is made in the antimesenteric surface of viable bowel just distal to the foreign body. The length of the incision approximates the diameter of the foreign body. The foreign body is delivered through the incision with gentle manual pressure.
Intestinal contents are milked 10 cm to either side of the foreign body and the bowel is held between an assistant’s fingers or with Doyen intestinal forceps. A No. 15 scalpel blade is used to make a full-thickness longitudinal incision in the antimesenteric border of the intestine in the viable tissue immediately proximal or distal to the foreign body. The length of the enterotomy approximates the diameter of the foreign body. Continuous suction is used to reduce spillage, and the surgeon pushes the foreign body gently through the enterotomy, taking care not to tear the incision margins (Figure 20-1B). The bowel lumen is examined for evidence of perforations or strictures before closure.
Linear foreign bodies such as string, fishing line, meat wrappers, and sewing yarn present a difficult surgical problem. The trailing end of a linear foreign body usually catches over the base of the tongue or in the pyloric antrum and acts as an anchor. Intestinal peristalsis attempts to move the foreign body distally, but because it remains fixed proximally, the bowel plicates itself along the length of the foreign body, which often cuts through the intestinal wall on the mesenteric surface, resulting in local peritonitis.
Linear foreign bodies should be managed by identifying the glossal anchor point initially and releasing it before laparotomy. Commonly, a gastrotomy is also necessary to free wadded string or fishing line from a gastropyloric anchor. The traditional way for linear foreign body removal requires multiple enterotomies to complete removal of the linear body (Figure 20-2). If too few enterotomies are made with too much traction placed on the linear body, the mesenteric border may be perforated in an area that is difficult to explore and suture. Occasionally, the intestinal foreign body perforates at several locations before surgery, and local peritonitis is evident. Sometimes, enough fibrosis has occurred around the foreign body so, even after its removal, the bowel retains its plicated conformation. In these patients, intestinal resection and anastomosis may be necessary.
Linear foreign body removal may often be facilitated using a urinary catheter technique. With this technique only one or two enterotomies are necessary. Once the foreign body is released from its proximal anchor point it is tied or sutured to the tip of an eight to 12 French vinyl urinary catheter (Figure 20-3A). The catheter tip is then pushed distally along the pleated length of bowel. As the catheter is pushed distally, the imbedded linear foreign body disengages from the intestinal wall (Figure 20-3B inset) and the bowel unpleats itself (Figure 20-3B). Once the foreign body is completely disengaged from the bowel wall a second short enterotomy is made distally over the distal tip of the catheter and the remainder of the foreign body is retrieved (Figure 20-3C). Alternatively a longer catheter can be used and pushed down through the colon. The foreign body can then be retrieved from the anus (not shown). The author has found catheter facilitated removal to be a very useful method for linear foreign body retrieval.
Closure of the enterotomy incision usually is performed with a simple interrupted suture pattern in side-to-side longitudinal fashion (Figure 20-4). Single-layer closures are recommended because double-layer closures may cause excessive narrowing of the lumen diameter. Various suture patterns are acceptable, but with all techniques, the vascular and collagen-rich submucosa must be incorporated in the sutures. Single-layer appositional techniques such as the simple interrupted appositional suture pattern is most commonly used. A simple interrupted approximating suture can be used (See Figure 20-10A). Sutures are placed 3 to 4 mm apart and 2 to 3 mm from the cut edge, taking care to incorporate all layers of the intestinal wall. Crushing sutures are tied tightly and cut through the muscularis and engage the submucosa. The author feels they should be avoided since they cause excessive hemorrhage and tissue ischemia (See Figure 20-10B). I prefer a modified Gambee suture, which incorporates the serosa, muscularis and submucosa but excludes the mucosa and is helpful in reducing mucosal eversion (See Figure 20-11).
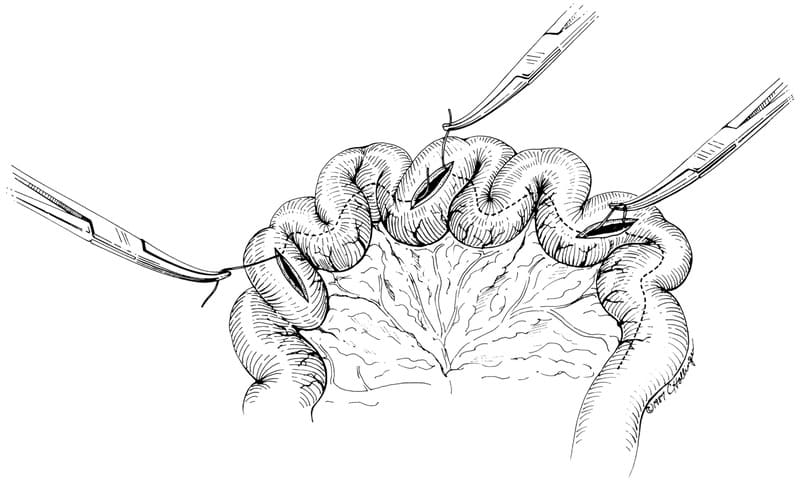
Figure 20-2. With a linear foreign body (e.g., a piece of string), multiple enterotomies usually are required. Mosquito hemostats are used to grasp a loop of the string at each enterotomy site. The string is then sequentially cut and withdrawn through the nearest enterotomy site. See text for details.



Figure 20-3. Fewer enterotomies are needed if A. The linear foreign body is tied or sutured to the tip of a urinary catheter. B. The catheter is pushed distally and disengages the foreign body from the intestinal mucosa (inset) as the intestine unpleats itself. C. A small enterotomy is made over the tip of the catheter and the foreign body is retrieved. If the catheter is long enough it can be pushed through the colon and out the anus (not shown).

Figure 20-4. An enterotomy usually is closed in side-to-side fashion interrupted suture pattern. Appositional, crushing, or modified Gambee sutures can be used.
The enterotomy also can be closed using a simple continuous approximating pattern (Figure 20-5). Suture bites are taken perpendicular to the bowe1 wall 2 to 3 mm from the cut edge and 3 mm apart. The suture line is advanced outside the bowel lumen. Sutures are pulled snugly enough to appose the wound edges gently. Pulling the suture line too tightly may cause strangulation of the wound edge and may lead to dehiscence. Some surgeons tend to close the enterotomy with a Cushing pattern. A continuous inverting Cushing pattern gives good serosa-toserosa apposition and luminal bursting strengths that exceed those of the interrupted approximating patterns for the first day post operatively. However, lumen diameter is reduced. Suture bites are placed 2 to 3 mm from the wound edge to minimize the amount of inversion (Figure 20-6). The tough submucosal layer is secured with each pass of the needle.
A rapid method of closing multiple enterotomies involves the use of a regular dimension skin stapler (AutoSuture multifire premium, United States Surgical, Norwalk, CT). Full thickness traction sutures are placed on both ends of the enterotomy and skin staples are placed every 2-3 mm (Figure 20-7). If the enterotomy is made in a small-diameter loop of bowel, longitudinal closure may cause luminal constriction. To prevent this constriction, the ends of the enterotomy can be closed in transverse fashion. A simple interrupted suture is used to approximate the proximal and distal ends of the longitudinal incision. Additional sutures are then placed 3 to 4 mm apart to appose the remaining bowel wall, resulting in a widened lumen diameter (Figure 20-8). For intestinal biopsies and for enterotomies in small animals I prefer to make a short transverse incision which goes not more than 30 to 40% around the diameter of the enterotomy and then close this wound transversely. I find that making the wound in this direction preserves lumen diameter better than either a longitudinal incision with side to side or transverse closure (Figure 20-9).
I prefer to close enterotomies with 3-0 to 4-0 synthetic monofilament suture material. Acceptable materials include polydioxanone (PDS, Ethicon, Inc.), poliglecaprone 25 (Monocryl, Ethicon, Inc.), polyglycomer 631 (Biosyn, United States Surgical) or polyglyconate (Maxon, United Status Surgical) on a narrowtaper, taper-cut, or small reverse-cutting need1e. Due to their rapid absorption time poliglecaprone 25 (Monocryl, Ethicon, Inc.) and polyglycomer 631 (Biosyn, United States Surgical) should be avoided in colonic surgery (See Chapter 1). Chromic surgical gut has been used with clinical success, but it is not recommended for intestinal closure because it loses tensile strength rapidly in the presence of collagenase and is quickly phagocytized in an infected environment. Nonabsorbable monofilament materials such as nylon (Ethicon, Ethicon, Inc.) or polypropylene (Prolene, Ethicon, Inc.) also may be used but foreign bodies have reportedly become attached to their exposed intraluminal segments. Stainless steel skin staples are reported to migrate into the lumen of the bowel and may be extruded in the feces. After the enterotomy closure is complete, it is rinsed with saline and covered with omentum (See Figure 20-19).

Figure 20-5. An enterotomy also can be closed with a simple continuous appositional pattern.

Figure 20-6. A continuous inverting Cushing suture pattern may be chosen for animals lumen who have a higher than normal risk of enterotomy leakage.

Figure 20-7. Multiple enterotomies can be closed in rapid fashion using a multifire skin stapler. Full thickness stay sutures are placed on the end of the wound and traction applied while staples are applied every 2-3 mm. (From Coolman BR, Ehrhart N, Marretta SM. Use of skin staples for rapid closure of gastrointestinal incisions in the treatment of canine linear foreign bodies. J Am Anim Hosp Assoc 36:542, 2000, with permission).

Figure 20-8. In a small-diameter loop of bowel, the longitudinal incision can be closed transversely to prevent luminal stenosis, a simple interrupted pattern is used.
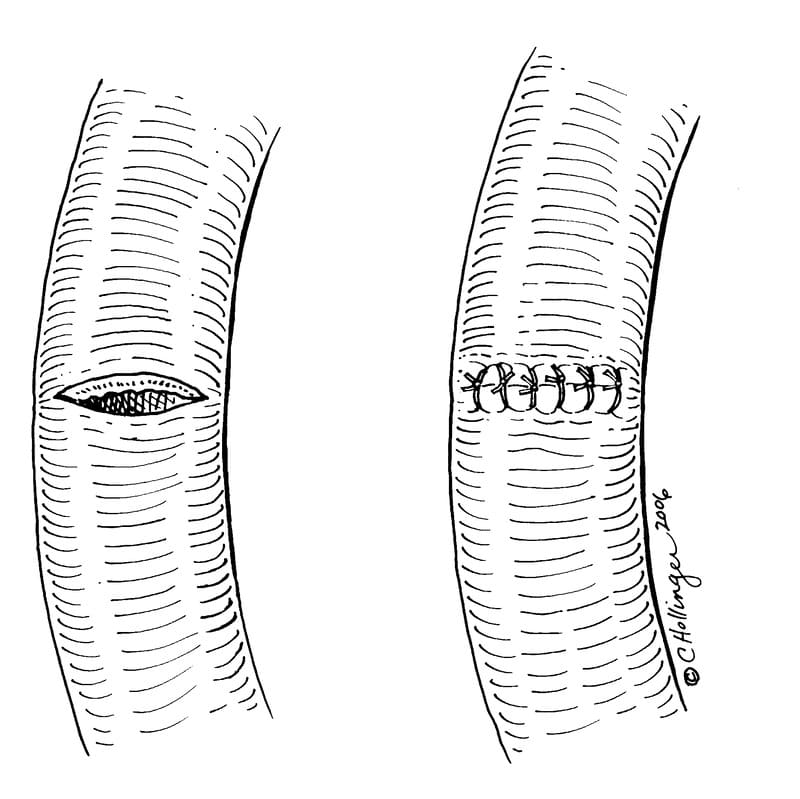
Figure 20-9. For intestinal biopsy make a transverse incision in the bowel wall and close the wound transversely with simple interrupted sutures to preserve lumen diameter.
Postoperative Care
The bacterial population of the small intestine is lowest in the proximal duodenum and highest in the distal ileum. Uncomplicated enterotomies of the proximal small bowel may not require postoperative antibiotic therapy. However, when spillage occurs or when an enterotomy is performed on the distal small bowel, parenteral antibiotics are administered prior to or during surgery and are continued for 24 to 48 hours postoperatively. Broadspectrum bactericidal agents such as intravenous cephazolin, at 10 mg/kg four times daily, in combination with enrofloxacin, 7.5 mg/kg IV twice daily, provide good prophylaxis against most gram positive cocci and gram-negative enteric organisms. Intravenous metronidazole, at 15 mg/kg PO four times daily, is also effective against anaerobic organisms.
Replacement intravenous fluids and electrolyte therapy are continued in the postoperative period until dehydration and acid-base and electrolyte abnormalities are resolved. Early introduction of food stimulates bowel contraction, reduces the likelihood of postoperative ileus or adhesion formation, and also serves as a valuable source of fluid and electrolytes. We begin feeding the day after surgery with small amounts of I/D gruel (Hills Pet Nutrition Inc., Topeka, KS). Persistent vomiting, fever, and leukocytosis in the presence of abdominal tenderness may indicate peritonitis resulting from leakage from the enterotomy. Abdominal paracentesis or diagnostic lavage should be performed. If a septic exudate is present, early exploration of the abdomen is indicated, and resection and anastomosis or one of the serosal patching techniques may be performed.
Suggested Readings
Anderson S, Lippincott CL, Gill PJ: Single enterotomy removal of gastrointestinal linear foreign bodies. J Am Anim Hospt Assoc 28:487, 1992.
Bebchuck TN: Feline gastrointestinal foreign bodies. Vet Clin N Am Sm Anim Pract 32(4):861, 2002.
Capak D, Simpraga M, Maticic D, et al: Incidence of foreign-body-induced ileus in dogs. Berliner un Munchener tierarztliche Wochenschrift 114(7-8):290, 2001.
Coolman BR, Ehrhart N, Marretta SM. Use of skin staples for rapid closure of gastrointestinal incisions in the treatment of canine linear foreign bodies. J Am Anim Hosp Assoc 36:542, 2000.
Ellison GW. Wound healing in the gastrointestinal tract. Semin Vet Med Surg 4:287, 1989.
Enquist IF, Bauman FG, Rehdcr E: Changes in body fluid spaces in dogs with intestinal obstruction. Surg Gynecol Obstet 127:17, 1968.
Fossum TW, Hedlund CS: Gastric and intestinal surgery. Vet Clin North Am Small Anim Pract 33(5):1117, 2003.
Kirpensteijn J, Maarschalkerweerd RJ, van der Gaag I, et al: Comparison of three closure methods and two absorbable suture materials for closure of jejunal enterotomy incisions in healthy dogs. Vet Q 23(2):67, 2001.
Mishra NK, Appert HE, Howard JM: The effects of distention and obstruction on the accumulation of fluid in the lumen of small bowel of dogs. Ann Surg 180:791, 1974.
Intestinal Resection and Anastomosis
Gary W. Ellison
Indications
Intestinal resection and anastomosis is performed for various common lesions of the small intestine. Mechanical obstructions, whether luminal, intramural, or extramural commonly require intestinal resection and anastomosis. Lodged intraluminal foreign bodies often cause local bowel wall necrosis or perforation, which may necessitate intestinal resection. Intramural lesions caused by strictures, neoplasms, or fungal granulomas caused by pythiosis must be removed by resection of the affected section of bowel. Occasionally, extramural lesions caused by adhesions secondary to previous surgery, regiona1 peritonitis, or abdominal abscesses require resection of the obstructed segment of intestine.
Strangulated loops of bowel associated with diaphragmatic, ventral, inguinal, perineal, or femoral triangle hernias often require emergency resection and anastomosis. Animals with intestinal or mesenteric volvulus have peracute mesenteric vascular pedicle obstruction and secondary bowel wall ischemia and may require massive resection and anastomosis. With intussusception, the invaginated segment of bowel undergoes early venous congestion and becomes edematous. Intussusceptions then become rapidly irreducible due to outpouring of fibrinous exudate from the invaginated serosal surface. If arterial thrombosis occurs, the invaginated bowel will become ischemic and necrotic. Resection and anastomosis of the affected section of bowel is then necessary.
Determining Intestinal Viability
Non-viable intestine is usually distended, blue, black or grey in appearance and easily discernable from normal bowel. In some cases, determining viability in cyanotic appearing bowel is difficult. The intestine should be decompressed with a needle and suction apparatus to relieve venous congestion. Standard clinical criteria for establishing intestinal viability are color, arterial pulsations, and the presence of peristalsis. Of these three parameters, peristalsis is the most dependable criteria of viability. The “pinch test” should be performed on questionable areas of bowel to determine whether smooth muscle contraction and peristalsis is present. If clinical criteria are inadequate to determine viability, intravenous fluorescein dye or surface oximetry can be used. A 10% fluorescein solution (Fundescein-10, Cooper Laboratories, San Germain, PR) is given at a dosage of 1 mL/5 kg intravenously through any peripheral vein. After 2 minutes, the tissues are examined using long-wave ultraviolet light (Wood’s lamp). Areas of bowel are considered viable if they have a bright green glow. Areas of bowel are not viable if they have a patchy density with areas of nonfluorescence exceeding 3 mm, have only perivascular fluorescence, or are completely nonfluorescent. Oxygen saturation may also be a reliable method of determining intestinal wall viability. A sterile probe is placed on the surface of the bowel and an oxygen saturation level reading will occur. According to published reports in rabbits, saturation levels of 81% or above typically mean that the bowel is viable. Values below 76% were consistent with mucosal necrosis and those below 64% indicated transmural intestinal necrosis.
Anastomotic Pattern and Suture Material
Although numerous suture techniques have been used for end-toend intestinal anastomosis in small animals, approximating patterns are recommended at present. Properly performed approximating techniques create a lumen diameter comparable to normal, result in rapid and precise primary intestinal healing, and minimize the potential for postoperative adhesion formation. Everting techniques (e.g., horizontal mattress pattern) initially create a larger lumen diameter, but ultimately they cause narrowing and stenosis of the lumen. Everting anastomoses are not recommended because they have a greater tendency to leak and because of delayed mucosal healing, prolonged inflammatory response, and increased adhesion formation compared with approximating anastomoses. Inverting anastomoses using Cushing or Connell patterns provide a temporary leak-resistant serosa-to-serosa approximation but they create an internal cuff of tissue, which may cause luminal stenosis. Inflammation is more severe and healing time is slower than with approximating techniques. Despite these dangers, inverting techniques should be considered in patients with a high risk of leakage or for use in colonic resection and anastomosis; in the latter situation, the high bacterial content of feces makes leakage of the anastomosis extremely dangerous.
Approximating end-to-end intestinal anastomoses can be created with various simple interrupted suture patterns or with a simple continuous suture pattern. Interrupted patterns generally are easier to perform, but the simple continuous pattern minimizes mucosal eversion and therefore provides better serosal apposition and primary intestinal healing. Regardless of the suture technique used, proper incorporation of the tough submucosa and reduction of mucosal eversion are vital in performing consistently successful intestinal anastomosis.
A simple interrupted appositional suture incorporates all tissue layers and gently apposes the wound edges (Figure 20-10A). A crushing suture is pulled tightly and cuts through the serosa, muscularis, and mucosa, and engages only the tough submucosal layer of the bowel wall (Figure 20-10B). Crushing sutures create more microhemorrhage and tissue necrosis directly at the anastomosis and the author feels they should be avoided. With both the appositional and crushing techniques, mucosal eversion tends to occur between sutures. I prefer a modified Gambee suture pattern because it reduces mucosal eversion. In this technique, the need1e is passed through the serosa, muscularis, and submucosa, but the mucosal layer is not incorporated in the suture (Figure 20-11). The suture is tied snugly enough to approximate all layers of the intestinal wall gently. The mucosa tends to be pushed into the intestinal lumen and does not evert between sutures.

Figure 20-10. A. Simple interrupted appositional suture, the wound edges are gently apposed. mu, mucosa; smu, submucosa; mus, muscularis; ser, serosa. B. Crushing suture. The knot is tied tightly cutting through all tissue layers and engaging the submucosa. This suture causes microvascular ischemia and tissue necrosis.

Figure 20-11. Modified Gambee suture. When tied, this suture gently approximates all tissue layers and slightly inverts the mucosa, thereby minimizing mucosal eversion between sutures (bottom). mu, mucosa; smu, submucosa; mus, muscularis; ser, serosa.
A taper-cut, narrow-taper, or small reverse-cutting need1e with 3-0 or 4-0 swaged-on suture material is suitable for most anastomoses. Braided, nonabsorbable materials such as silk or braided polyesters should be avoided. Chromic surgical gut rapidly loses tensile strength due to collagenase and phagocytosis at the wound edge and is not recommended. Synthetic, braided, absorbable suture materials such as polyglactin 910 (Vicryl, Ethicon, Inc., Somerville, NJ) are acceptable, but they have significant tissue drag. I prefer poliglecaprone 25 (Monocryl, Ethicon Inc., Somerville, NJ), glycomer 631 (Biosyn, United States Surgical Corp, Norwalk, CT), polydioxanone (PDS, Ethicon, Inc., Somerville, NJ), and polyglyconate (Maxon, United States Surgical Corp., Norwalk, CT), which are monofilament absorbable sutures with little tissue drag and have all been used successfully for intestinal anastomoses. Nonabsorbable monofilament sutures such as nylon (Ethicon, Ethicon, Inc., Somerville, NJ) or polypropylene (Prolene, Ethicon, Inc., Somerville, NJ) also are acceptable for simple interrupted anastomoses, but they should not be used for simple continuous anastomoses because they do not allow luminal distension. Newer versions of triclosan impregnated polygalactin 910 (Vicryl plus, Ethicon Inc., Somerville, NJ) and poliglecaprone 25 (Monocryl plus, Ethicon Inc., Somerville, NJ) are undergoing investigation in hopes that this bacteriostatic compound will reduce wound infection.
Surgical Technique
A standard midline laparotomy is performed, as well as a thorough examination of the intestinal tract. The area to be resected is packed away from the abdomen with moistened laparotomy sponges. Intestinal contents are milked proximally and distally, and the bowel is held between an assistant’s index fingers or with Doyen intestinal forceps 4 to 5 cm from the proposed resection site. A 1- to 2-cm margin of normal viable intestine is included in the proximal and distal boundaries of the area to be resected, which is clamped with Carmalt or Doyen forceps. If luminal disparity is present, the forceps are placed at a 75° to 90° angle on the dilated proximal segment (Figure 20-12A) and at a 45° to 60° angle on the contracted distal segment of bowel (Figure 20-12B). Branches of the mesenteric artery and veins supplying the devitalized bowel are isolated with curved mosquito forceps and are double-ligated. The arcadial vessels located within the mesenteric fat are double-ligated at the area of the proposed resection. A scalpel blade is used to excise the bowel along the outside of the intestinal forceps (See Figure 20-12, dashed lines). With dissecting scissors, the vessels are divided, the mesentery is transected (See Figure 20-10, dotted lines), and the excised bowel is removed from the surgical field. After resection, the small intestinal mucosa has a tendency to evert and can be trimmed back with Metzenbaum scissors (Figure 20-13).
If angling the intestinal incision does not adequately correct for luminal disparity, the smaller stoma can be enlarged by incising the bowel section for a distance of 1 to 2 cm along the antimesenteric surface and then trimming off two triangular flaps (Figure 20-14). This procedure creates an ovoid larger stoma, which can be anastomosed to the larger-diameter section of the bowel.
When the anastomosis is closed with a simple interrupted suture technique, the first suture is placed at the mesenteric border because the presence of fat in this area makes suture placement most difficult, and this is where leakage is most likely to occur. The second suture is placed on the antimesenteric border, and the third and fourth sutures are placed laterally at the 90° quadrants (Figure 20-15A). Depending on bowel diameter, two to four more sutures are placed between each of the quadrant sutures (Figure 20-15B). All sutures are placed 3 to 4 mm apart and 2 to 3 mm from the wound edge. Suture bites on the dilated side of the anastomosis are placed farther apart than on the contracted side of the anastomosis to correct for luminal disparity. Once one side of the anastomosis is sutured, the bowel is flipped over, and the opposite side is completed. From 12 to 20 sutures are used to complete the anastomosis. After the anastomosis has been completed, it is checked for leakage by infusing saline under low pressure into the bowel lumen and massaging the fluid past the anastomosis. The anastomosis can also be checked by gently probing the spaces between sutures with mosquito hemostats for openings. The surgeon then closes the mesenteric defect with a simple continuous pattern, taking care not to include any mesenteric vessels within the suture line (Figure 20-15C).
Occasionally, the small-diameter loop of bowel cannot be enlarged enough to be anastomosed to the larger one. In this case, the large-diameter stoma is reduced by initially angling the cut at 45°. The anti-mesenteric portion of the incision is then apposed with simple interrupted sutures in side-to-side fashion until the remaining opening is an appropriate width to anastomose to the smaller-diameter loop of bowel (Figure 20-16).

Figure 20-12. Proximal A. and distal B. forceps are placed at the area to be resected. Mesenteric and arcadial vessels are double-ligated as shown. The bowel is transected with a scalpel blade outside of the clamps (dashed lines), and the mesentery is incised with dissecting scissors (dotted lines). See text for details.

Figure 20-13. Everted mucosa can be trimmed back before the anastomosis is performed.

Figure 20-14. Enlargement of bowel section with a smaller diameter may be necessary prior to anastomosis. See text for details.

Figure 20-15. Closing anastomosis with simple interrupted suture pattern. A. Placement of first (1), second (2), and third (3) sutures; the fourth suture is placed on the lateral bowel wall opposite to the third suture. B. Additional sutures are placed between each of the original four. C. Final step is closure of the mesenteric defect with simple continuous sutures. See text for details.

Figure 20-16. Lumen diameter of larger stoma can be reduced to equal that of smaller diameter (top), so anastomosis can be completed (bottom). See text for details.
Alternatively, a simple continuous approximating technique can be used to create the anastomosis. This is performed with two lengths of suture. The first knot is tied at the mesenteric border and the second at the antimesenteric border (Figure 20-17A). The sutures are then advanced around the perimeter approximately 3 mm from the cut edge, with the wound edges gently approximated. The needles are advanced in opposite directions, so one knot is tied to the tag at the antimesenteric border. The final knot is tied to the tag on the mesenteric border (Figure 20-17B and C). If the knot is tied too tightly, a pursestring effect will be produced, and stenosis of the anastomosis may occur. The completed anastomosis is tested for leakage, and the mesenteric defect is closed.
A rapid alterative to sutured anastomosis is the use of stainless steel skin staples. Three stay sutures are used to triangulate the bowel ends and an end-to-end anastomosis is performed with an AutoSuture 35 skin stapler with stainless skin staples (United States Surgical Corp., Norwalk, CT). After triangulating the intestine with three stay sutures, the skin stapler is used to place staples every 2-3 mm around the perimeter of the wound (Figure 20-18). These closures are more rapidly done than handsewn anastomosis and have similar bursting strengths, but some mucosal eversion is created.
Leakage of any intestinal anastomosis is most common in animals with pre-existing peritonitis, low serum albumin and in those animals where intestinal foreign bodies have created intestinal ischemia. To help prevent anastomotic leakage, a pedicle of greater omentum is wrapped around the suture line. The omentum is critical to the successful healing of intestinal wounds because it can seal small anastomotic leaks and can prevent peritonitis. Dogs with the greater omentum removed have significant morbidity and mortality associated with intestinal anastomosis, whereas most dogs survive and do well when the omentum is retained. The omentum is tacked to the serosa with two simple interrupted sutures of 3-0 suture material placed on each side of the bowel wall (Figure 20-19).

Figure 20-17. Intestinal anastomosis using the simple continuous approximating suture pattern. Two lengths of suture are used. A. The first knot is tied at the mesenteric border and the second at the antimesenteric border. B. The sutures are advanced in opposite directions around the perimeter of the bowel. C. Knots are tied to tags at the mesentery and antimesentery. See text for details.
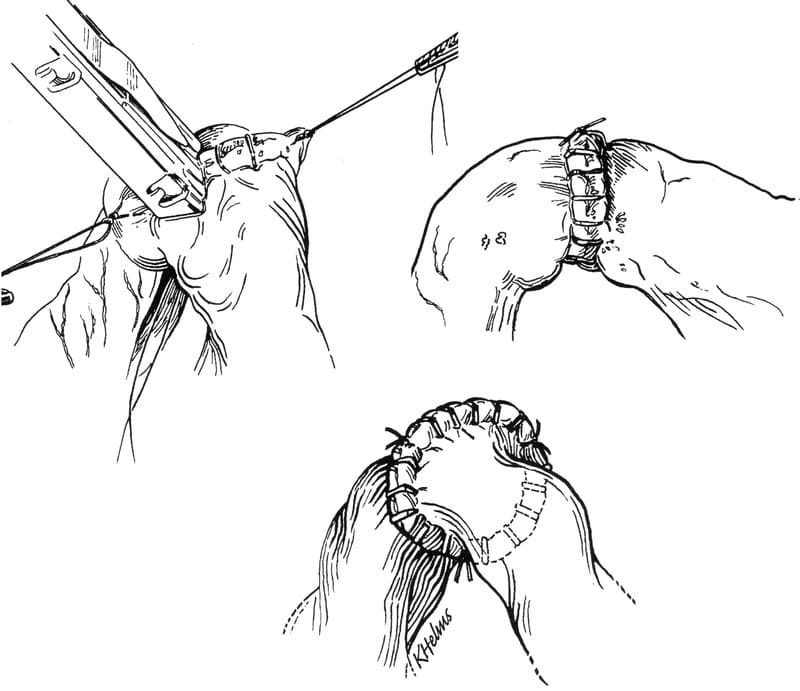
Figure 20-18. Anastomosis can be fashioned using skin staplers by first triangulating the wound ends and then applying staples every 3 mm around the perimeter of the anastomosis. (From Coolman BR, Ehrhart N, Pijanowski G, et al. Comparison of skin staples with sutures for anastomosis of the small intestine of dogs. Vet Surg 29:293, 2000, with permission).

Figure 20-19. To help prevent leakage a pedicle of greater omentum is wrapped around all enterotomies and anastomoses and is tacked to the serosa on both sides with simple interrupted sutures.
Postoperative Care
Fluid and electrolyte deficits are corrected and antibiotic therapy is continued in the postoperative period. The author uses metoclopramide 2.2 mg/kg IV every eight hours to reduce ileus and promote intestinal motility. Feeding a bland diet such as canned I/D gruel (Hills Pet Nutrition Inc., Topeka, KS) is initiated the day following surgery. In uncomplicated cases, reasonable appetite usually resumes within 48 hours. Anorexia or vomiting in the presence of fever, abdominal tenderness, and leukocytosis suggests that anastomotic leakage and peritonitis may have occurred. If degenerate neutrophils with engulfed bacteria or free peritoneal bacteria are present on abdominocentesis, early reexploration of the abdomen is warranted. Further resection and reanastomosis or use of one of the serosal patching techniques described later in this section may be required. Aggressive treatment of generalized peritonitis may be needed to salvage the patient.
Managing Animals with Massive Resection
The propensity for short-bowel syndrome after massive intestinal resection depends on the amount of tissue excised, the location of the resection, and the time allowed for adaptation. Resection of up to 80% of the small intestine in puppies may allow for normal weight gain, whereas resection of 90% produces morbidity and mortality. After resection of large portions of small intestine, maldigestion, malabsorption, diarrhea induced by fatty acids or bile salts, bacterial overgrowth, and gastric hypersecretion may occur. Location of the resection is important. High resection of the duodenum and upper jejunum may decrease pancreatic enzyme secretion because pancreatic-stimulating hormones such as secretin and cholecystokinin are produced in the mucosa of these sections. These reductions in release of pancreatic enzymes contribute to maldigestion. Maldigestion of protein, carbohydrate, and fat leads lo catabolism, negative nitrogen balance, and steatorrhea. Unabsorbed sugars also may cause osmotic diarrhea. If the ileocecal valve is resected, bacteria may ascend, overgrow in the small bowel, and contribute to diarrhea.
After massive resection, the remaining small intestine adapts by increasing lumen diameter, enlarging microvilli size, and increasing mucosal cell number. These compensatory changes may take several weeks; during this period, parenteral fluids, electrolytes, and hyperalimentation may be necessary for the survival of the animal. During this time, the animal ideally will be able to maintain weight even with diarrhea. Medical treatments for unresponsive diarrhea after massive resection include frequent small meals, low-fat diets such as intestinal diet (I/D Hills, Topeka, KS) elemental diet supplements, medium-chain triglyceride oils, pancreatic enzyme supplements, B vitamins, kaolin antidiarrheals, and poorly absorbed oral antibiotics such as neomycin.
Suggested Readings
Agrodnia M, Hauptman J, Walshaw R. Use of atropine to reduce mucosal eversion during intestinal resection and anastomosis in the dog. Vet Surg 32(4):365, 2003.
Bone DL, Duckett KE, Patton CS, et al: Evaluation of anastomosis of small intestine in dogs: crushing versus noncrushing suturing techniques. Am J Vet Res 44:2043, 1983.
Chatworthy HW, Saleby R, Lovingood C: Extensive small bowel resection in young dogs: its effect on growth and development. Surg 32:341, 1952.
Coolman BR, Ehrhart N, Pijanowski G, et al: Comparison of skin staples with sutures for anastomosis of the small intestine of dogs. Vet Surg 29:392, 2000.
Crowe DT: Diagnostic abdominal paracentesis techniques: clinical evaluation in 129 dogs and cats. J Am Anim Hosp Assoc 20:223, 1984. Ellison GW: End to end intestinal anastomosis in the dog: a comparison of techniques. Comp Cont Educ Sm Anim Pract 3:486, 1981.
Ellison GW, Jokinen MC, Park RD: End to end intestinal anastomosis in the dog: a comparative fluorescein dye, angiographic and histopathologic evaluation. J Am Anim Hosp Assoc 18:729, 1982.
Erikoglu M, Kaynak A, Beyatli EA, et al: Intraoperative determination of intestinal viability: a comparison with transserosal pulse oximetry and histopathological examination. J Surg Res 128(1):66, 2005.
Krahwinkel DJ, Richardson DC: Intestines. In: Bojrab MJ, ed. Current techniques in small animal surgery. 2nd ed. Philadelphia: Lea & Febiger, 1983.
McLackin AD: Omental protection of intestinal anastomosis. Am J Surg 125:134, 1973. Ralphs SC, Jessen CR, Lipowitz AJ. Risk factors for leakage following intestinal anastomosis in dogs and cats: 115 cases (1991-2000). J Am Vet Med Assoc 223(1):73, 2003.
Weisman DL, Smeak DD, Birchard SJ, et al: Comparison of a continuous suture pattern with a simple interrupted pattern for enteric closure in dogs and cats: 83 cases (1991-1997). J Am Vet Med Assoc 214(10):1507, 1999.
Wheaton LB, Strandberg JD, Hamilton SR, et al: A comparison of three techniques for intraoperative prediction of small intestinal injury. J Am Anim Hosp Assoc 19:897, 1983.
Subtotal Colectomy in the Cat and Dog
Ron M. Bright
Introduction
Megacolon is defined as distension of the large intestine that is usually associated with various degrees of colonic hypomotility. In the cat and dog, this is usually an acquired disorder related to mechanical obstruction of the rectum or colon due to a foreign body, dietary indiscretion, neoplasia (intraluminal/extraluminal), and malformation and stenosis of the pelvis secondary to a healed pelvic fracture. Neurological deficits associated with lumbosacral disease or dysautonomia, a progressive polyneuropathy of the autonomic nervous system of older cats, can also lead to megacolon. Manx cats with partial or complete absence of the sacral and caudal spinal cord may have megacolon with concurrent urinary or fecal incontinence. In cats, megacolon is considered an idiopathic disorder in the majority of cases. Megacolon usually results in impaction of feces resulting in constipation or obstipation.
Dogs and cats can have constipation for several days without clinical signs. If obstruction of the movement of feces is delayed, the stool becomes harder and can form concretions. This retention of feces, if chronic or prolonged, can result in severe distention of the colon and motility disorders. It can also result in various degrees of mucosal injury that may result in absorption of bacterial toxins contributing to more severe clinical signs. The duration of obstruction that leads to more severe mucosal changes is unknown. One study in cats suggests that if colonic distension is present for 6 months or longer as may be seen with pelvic stenosis secondary to pelvic fractures, degenerative intramural myoneural changes in the colon may not allow return to normal function even if the cause of obstruction is relieved.
When constipation progresses to obstipation, excessively hard feces will prevent defecation. Digital removal of the impaction is usually necessary in these cases. When the condition progresses to obstipation, medical therapy becomes ineffective.
History and Clinical Signs
Regardless of the cause of the constipation, tenesmus with little or no production of feces is the most common complaint. It is not uncommon to have passage of mucus and/or blood associated with obstipation as a result of inflammation of the colonic mucosa. Historically, the owner may describe a possible etiology such as pelvic or lumbosacral trauma or dietary indiscretion.
Systemic signs depend on the duration of the obstipation and degree of injury to the colonic mucosa. These signs can include anorexia, weight loss, lethargy, dehydration, vomiting, and liquid bloody feces. Some cats will eventually become unthrifty and have perineal soiling. Hard concretions within an enlarged colon will often be palpated and some discomfort may be noted. Rectal palpation is done to evaluate for any pelvic canal stenosis, the presence of a perineal hernia, and any intraluminal or extraluminal masses that can result in a mechanical obstruction. In the dog, prostatomegaly or severe lymphadenomegaly of the iliac/ sublumbar lymph nodes associated with neoplasia should be considered and carefully evaluated.
Diagnosis
Tenesmus and decreased fecal production should prompt the clinician to consider constipation/obstipation secondary to megacolon. Abdominal and pelvic radiographs will help confirm megacolon and may identify pelvic abnormalities or lumbosacral disease, or other abdominal masses that may be causing colonic or rectal obstruction.
Careful palpation of the abdomen should be performed after feces has been evacuated. Ultrasound examination or colonoscopy can be used to rule out other disease processes such as neoplasia or stricture, especially if there are palpable abnormalites.
Barium enema contrast studies of the rectum and colon may be valuable and can be performed especially in dogs after evacuation of the feces.
Conservative Treatment
Medical management is indicated prior to any surgical intervention. Warm water enemas followed by laxatives and dietary supplements (canned pumpkin) may be helpful. Cisapride, has been used successfully to stimulate colonic motility (0.25 mg/ kg or 2.5 mg every 8-12 hours for smaller cats and 5 to 10 mg every 8 to 12 hours in larger cats and dogs). This dose can be safely doubled if lower doses are not effective. Cisapride is no longer commercially available but some pharmacies are able to compound this drug on request. I prefer to use cisapride and lactulose (Lactulose generic, Apotex) concurrently to optimize the effect of keeping the colon evacuated. Some cats aggressively treated in this manner may never require surgical intervention. Other cats, however become less responsive to medical management over time and require surgery.
Surgical Treatment
A subtotal colectomy was once considered a “salvage” procedure. However, a long-term history of success with this technique makes it a very good alternative to medical therapy. Surgery is most often performed in those patients who fail to respond to aggressive medical therapy. However, I have had several owners that opt for surgery on their cat because of their unwillingness or inability to be involved in medical management, which becomes cumbersome or causes behavior problems with the cat. Another group of owners eventually select a surgical option because of the emotional cost that is associated with restraining their cat and giving the appropriate medications.
In cats, a bilateral perineal hernia may be seen concurrently with megacolon. In these cases, performing a subtotal colectomy is usually sufficient to relieve the signs. If not, a bilateral herniorrhaphy may be necessary at a later time.
The standard of surgical treatment for megacolon in the cat is a subtotal colectomy that involves removal of approximately 95% of the colon. I prefer preservation of the ileocolic valve (ICV) in most cats and in all dogs, although numerous reports cite good results when the ICV is removed in cats. I do not remove the ICV except in those cats where a colocolostomy will result in too much tension across the anastomosis.
Before an animal has colectomy performed, it should be carefully evaluated for concurrent problems that may detract from a successful outcome. Loss of anal sphincter tone that is not diagnosed prior to a subtotal colectomy will usually result in an unsatisfactory outcome. Rectal stricture or neoplasia should be ruled out by performing a digital rectal examination prior to surgery.
If the megacolon is the result of an acquired pelvic stenosis that is the result of pelvic fracture malunion and it is less than 6 months from the time of injury, a hemipelvectomy or corrective osteotomy can be tried. The technical demands of the orthopedic procedures make the subtotal colectomy a more viable option.
Enemas should not be administered within 48 hours of surgery to decrease the risk of contamination from liquid intestinal contents at the time of surgery. Applying aseptic surgical principles to colonic surgery, carefully isolating segments of bowel with saline-soaked laparotomy sponges or towels, and employing meticulous and gentle handling of tissues will help ensure success. Perioperative use of an appropriate antimicrobial drug is indicated because the surgery results in a “clean contaminated” or “contaminated” wound. A broad-spectrum antibiotic such as a second-generation cephalosporin such as cefoxitin (Mefoxin, Merck and Co.) is preferred because of its effectiveness against most anaerobes as well as the usual gram-negative aerobes. It is preferable to give the drug preoperatively intravenously. Administering the drug 20 to 30 minutes prior to surgery at a dose of 20 mg/kg will result in optimum blood levels of the drug at the operative site. This is repeated 2 to 3 hours later.
Subtotal colectomy is performed through a ventral midline abdominal incision extending from the umbilicus to the pubis. The appropriate colic and caudal mesenteric vessels are ligated and divided. (Figure 20-20) If the ICV is resected, then additional ligatures are necessary for the ileocecocolic artery and vein. I do not find it necessary to ligate the cranial rectal vessels.
In order to optimize exposure of the colon and the planned site of anastomosis, it is helpful to exteriorize the small bowel from the abdomen to the right of the abdominal incision. Moistened laparotomy pads are placed to protect and moisten the small intesine. The urinary bladder is emptied manually or by cystocentesis to ease isolation from the surgical site. Fecal material is massaged toward the middle of the segment of the colon to be removed away from the site of intestinal transection. The colon or ileum is transected proximally and again distally 1-2 cm rostral to the pubis. Straight intestinal clamps (Doyen) are used to hold the segments of the bowel together during the anastomosis. I prefer to perform a single layer anastomosis using simple interrupted appositional sutures of 4/0 polydioxanone or polypropylene (Prolene and PDS, Ethicon, Inc., Somerville, NJ). Some cats have concurrent inflammatory bowel disease and a biopsy of the small bowel may be indicated.
When preserving the ICV, a 2-3 cm segment of the proximal colon is preserved and anastomosed to the 1 to 2 cm segment of remaining distal colon just ahead of the pubic bone (Figure 20-21). Holding these segments together during the suturing process requires intestinal forceps. If there is lumen disparity between the two segments as when the ICV is resected, then the smaller lumen (ileum) can be spatulated to increase its circumference to match that of the opposite larger colonic segment (See Figure 20-14). Alternatively, the larger lumen segment can be oversewn until it matches the diameter of the smaller segment and the anastomosis is completed with a simple interrupted approximating suture pattern using 3 or 4-0 suture size (Figure 20-22). Following the anastomosis, an attempt is made to remove any remaining feces from the rectum by massaging the material distally followed by digital removal through the anus at the conclusion of surgery.
In lieu of the standard suturing technique for the anastomosis, a surgical stapler may be used with the placement via the rectum or transcecally (EEA stapler, U S Surgical). Recently a single-use biofragmentable anastomosis ring, BAR, (Valtrac, US Surgical) has been described and compared to conventional suture technique for restoration of bowel continuity. The BAR is a sutureless inverting anastomosis technique that has compared favorably with standard anastomosis techniques.
Following subtotal colectomy, tenesmus and/or hematochezia may be observed. This usually resolves within 7-10 days. A soft stool will be present indefinitely following this surgery and it appears that the return to a somewhat normal consistency occurs sooner when the ICV is preserved. Frequency of defecation usually increases and rarely is anastomotic stricture a problem postoperatively. Balloon dilation and the use of laxatives have been successful in treating stricture when it has occurred.
Most cats are greatly improved following a subtotal colectomy with normal bowel function. The need for medical management is unlikely. Some cats may continue to have bloody diarrhea and various degrees of discomfort when defecating. This may be related to a stricture at the site of anastomois or inflammatory bowel disease. Endoscopy and biopsy are required to confirm the etiology. Medical therapy is usually successful in improving signs related to inflammatory bowel disease and balloon dilatation and laxatives are usually successful in reversing signs related to stricture.
I and others have used subtotal colectomy in dogs successfully. The most common indication is for pelvic malformation. Dietary discretion has also been the cause of megacolon in one dog. The prognosis is good in dogs but it appears that preservation of the ICV is much more important in the dog. Their ability to adapt to the absence of the colon and the ICV seems inferior to the cat.

Figure 20-20. The appropriate colic and caudal mesenteric vessels (arrows) are ligated before division of the colon. If the ileocolic valve is removed, the ileocecocolic vessels (open arrow) need to be ligated as well. With the ileocolic valve preserved (my preference), a small length (I to 2 cm) of ascending colon remains after transecting the bowel A. Likewise, when transecting the distal colon B. a small remnant of colon or cranial rectum is left to anastomose to the proximal segment.

Figure 20-21. The mesenteric sides of the proximal A. and distal B. bowel segments are aligned before proceeding with the anastomosis.

Figure 20-22. When lumen disparity exists between the two segments to be anastomosed, the larger lumen can be sutured closed until the remaining lumen approximates the size of the opposite segment.
Suggested Readings
Bertoy RW: Megacolon In Bojrab MJ, ed.: Disease mechanisms in small animal surgery. 2nd ed. Philadelphia: Lea and Febiger, 1993, p 262.
Bright RM: Subtotal colectomy for treatment of acquired megacolon in the dog and cat. J AM Vet Med Assoc 12: 1412, 1986.
DeNovo RC, Bright RM: Chronic feline constipation/obstipation. In Kirk RW, Bonagura JD, eds. Current Veterinary Therapy XI. Philadelphia: WB Saunders, 1992, p 619.
Hoskins JD. Management of feline impaction. Compend Contin Educ Pract Vet 12: 1579, 1990.
Kudish M, Pavleteic MM: Subtotal colectomy with surgical stapling instruments via a transcecal approach or treatment of acquired megacolon in cats. Vet Surg 22: 457, 1993.
Matthiesen DT, Scavelli TD, Whitney WO. Subtotal colectomy for the treatment of obstipation secondary to pelvic fracture malunion in cats. Vet Surg 20: 113, 1991.
Ryan, S. Comparison of a biogragmentable anastomosis ring and sutured anastomosis for subtotal colectomy in cats with megacolon. Proceedings of the 4th Annual Scientific Meeting of the Society for Veterinary Soft Tissue Surgery. June 2005.
Pozzi A, Smeak DM. Subtotal colectomy in the dog. Personal communication, 2005.
Surgery of the Colon and Rectum
Brian T. Huss
This topic is written based on the available literature through 2010 and does not cover the most current literature on this topic.
Introduction
Colorectal surgery in small animals can be performed with the same surgical success rates as other gastrointestinal surgery with the use of careful tissue handling techniques and modern surgical materials.
The large intestine of the dog and cat is shorter than the small intestine, ranging from approximately 20 to 35 cm in length.1,2 As a general rule, the large intestine is approximately the length of the trunk in dogs and cats, with the small intestine measuring about four times the length of the trunk. Because of its shorter mesentery, the large intestine does not vary as much in length or position as the small intestine. The large intestine is, however, considerably larger in internal diameter than the small intestine, and has neither the tenia (longitudinal bands) nor haustra (sacculations) seen in other species. Classically, the large intestine has been divided into the cecum, colon (ascending, transverse, and descending), and rectum (Figure 20-23).
Microscopically, the colon is composed of five layers. From the inner luminal surface outward the layers of the colon are 1) mucosa, 2) submucosa, 3) circular muscle layer, 4) longitudinal muscle layer, and 5) serosa. The mucosa consists of columnar epithelial lining cells, mucus secreting goblet cells, and enteroendocrine cells. Intestinal villi are absent in the colonic mucosa; however, intestinal crypts (crypts of Lieberk¸hn) remain. Intestinal crypts are elongated and straight, opening onto the luminal surface of the colon. The submucosa is composed of collagen and elastin fibers arranged in an orderly honeycomb pattern, with submucosal glands and lymphoid tissue dispersed throughout this layer. The submucosa’s high collagen and elastin content makes it the important suture holding layer of the intestine. Tunica muscularis is the term commonly given the combined smooth muscle layers of the intestine. Contraction of this group of muscles is responsible for intestinal motility. Finally, the tunica serosa consists of loose connective tissue covered with a layer of squamous mesothelial cells.
The large intestine is anchored to the sublumbar region by the mesocolon, which arises from the left side of the mesentery and is divided into the same parts as the colon that it suspends. The blood supply to the colon and rectum arises from the cranial and caudal mesenteric arteries supported in the mesocolon (See Figure 20-23). The cranial mesenteric artery supplies the cecum, ascending, transverse, and part of the descending colon. The caudal mesenteric artery supplies the remainder of the descending colon as well as the rectum.1-4 Numerous perpendicular branches (vasa recta) split from the colic arteries, anastomosing with each other along the lesser curvature of the colon. Most of the large intestine is drained by the portal system through the ileocolic and caudal mesenteric veins.1-4 The caudal rectal vein drains the anal canal and empties directly into the caudal vena cava.1-4

Figure 20-23. Surgical anatomy of the feline large intestine, ventral view. Legend: A-jejunum, B-ileum, C-cecum, D-ascending colon, E-transverse colon, F-descending colon, G-mesentery, H-ileocecal fold, I-mesocolon, J-caudate process of liver, K-right kidney, L-right ureter, M-caudal mesenteric lymph nodes, 1-abdominal aorta, 2-caudal vena cava, 3-cranial mesenteric a., 4-jejunal a., 5-ileal a., 6-ileocolic a., 7-colic branch, 8-cecal a., 9-antimesenteric ileal branch, 10-ileal mesenteric branch, 11-right colic a., 12-middle colic a., 13-left renal vessels, 14-testicular a., 15-caudal mesenteric a., 16-left colic a., 17-cranial rectal a., 18-middle colic v.
Indications for Surgery
The need for colonic surgery in small animals is not as common as the need for small intestinal surgery. Colonic surgery techniques involve primary closure of traumatic defects, resection and anastomosis, biopsies, and rarely, foreign body removal.
Trauma to the colon can result from intraluminal or extraluminal sources. Intraluminal causes of injury are rare, but such injury can result from ingested sharp foreign bodies or improper use of transanal instruments. Colonic foreign bodies can often be gently milked through the colon to a point at which they can be grasped by an assistant using a transanal forceps. Rarely, a colotomy must be performed to retrieve a foreign body. Extraluminal sources of trauma are more common and include gunshot and knife wounds, and less commonly, penetrating bone fragments from pelvic fractures. Indirect or blunt trauma to the colon can also result in contusions, vessel thrombosis, colonic torsion, or even avulsions of the colon. Penetrating wounds of the colon require immediate treatment. Primary repair of clean lacerations, debridement and primary closure of more severe wounds, or resection and anastomosis of devitalized segments may be required to close colonic defects. In one study of dogs with rectal tears resulting from pelvic fractures, only dogs with tears repaired within 24 hours of trauma survived.5
Neoplasia of the colon is less common than in other parts of the alimentary system. Benign tumors of the colon commonly include leiomyomas, papillary adenomas, and adenomatous polyps. Malignant transformation of adenomatous polyps has been reported to occur in 18% of dogs in one study.6 Malignant tumors of the colon commonly include lymphosarcomas, carcinomas, and adenocarcinomas. Metastasis of colonic tumors occurs most commonly to the regional lymph nodes and the liver. Intussusception of the large intestine occurs most commonly at the ileocecocolic junction. Intussusception of the body of the colon is rare. Intussusceptions of the large intestine are treated in the same manor as those occurring in the small intestine.
Colectomy, either partial or complete, may be the treatment of choice for patients with unresponsive megacolon, severe unresponsive inflammatory bowel disease, colonic ulcerations, colonic strictures, colonic torsion, and pelvic canal stenosis resulting from pelvic fracture malunion. Removal of the cecocolic valve has been advocated in the case of megacolon caused by pelvic fracture malunion, to create a soft stool. Most surgeons, however, recommend leaving the cecocolic valve in the treatment of other colonic diseases.
Surgical biopsy of the colon may be the diagnostic method of choice in some colonic diseases. Direct visualization of the entire colon, the ability to safely obtain multiple full thickness samples of colonic wall and regional lymph nodes, and commonly available surgical instrumentation make open colonic biopsy a viable diagnostic method.
Diagnostic Methods
Diagnosis of colorectal disease is based upon physical exam findings and various imaging techniques. Colonic masses can often be palpated in the central to caudal aspect of the abdomen. Rectal masses can often be felt upon digital rectal examination. Survey abdominal and pelvic radiographs are recommended in all patients with suspected large intestinal disease. Radiographs can give indications of regional lymph node size, luminal contents, including the degree of colonic filling and overall density of the luminal contents. Radiographs can also help to diagnose intraluminal or extraluminal foreign bodies, or space occupying lesions, and they give a rough estimate of intestinal wall thickness, as well as, plication or intussusceptions of the intestine. However, abdominal ultrasound provides a better view of the intestinal wall and has become the imaging method of choice for diagnosing intussusceptions. Ultrasonography also allows more detailed imaging of intra-abdominal structures when peritonitis is present and for biopsy and staging of patients with neoplasia. Positive-contrast enemas may be helpful diagnostic tools in selected cases; however, they are contraindicated when perforations or weakened intestinal walls are suspected.
Other diagnostic methods that may be of benefit in large intestinal diseases are proctoscopy, computed tomography (CT) scans and magnetic resonance imaging (MRI). Proctoscopy should be performed with care if weakened intestinal walls are suspected and it is contraindicated when large intestinal perforations are suspected. Computed tomography scans and MRI are most useful when staging patients with cancer to determine the extent and spread of disease.
Microscopic analysis of peritoneal fluid can provide a definitive diagnosis in the case of intestinal perforation. Fluid can be obtained via abdominal paracentesis or, ideally, by peritoneal lavage. A large number of neutrophils with intracellular bacteria are diagnostic of bacterial peritonitis. Less definitive are fluid samples with large numbers of degenerative neutrophils, free abdominal bacteria, or debris which would normally be found intraluminally. Inadvertent sampling of the intestinal lumen could account for these findings. A peritoneal lavage is recommended to confirm equivocal results.
Preoperative Preparation
Bacterial populations in the normal gastrointestinal tract increase dramatically from oral to aboral, changing from predominately aerobic to predominately anaerobic. A gram of feces from the colon contains up to 1011 organisms.7 Aerobic bacteria in the large intestine normally include the Gram-positive genera Streptococcus, Staphylococcus, Bacillus, and Corynebacterium and Gram-negative members of the enterobacter family, especially Escherichia coli, Enterobacter, Klebsiella, Pseudomonas, Neisseria, and Moraxella. 7 Up to 90% of the bacteria in the large intestine are anaerobes, including members of the Gram-positive genera Clostridium, Lactobacillus, Propionibacterium, and Bifidobacterium; the Gram-negative anaerobic bacteria include Bacteroides, Fusobacterium and Veillonella. 7 The importance of anaerobic bacteria as pathogens in small animals, especially Bacteroides fragilis, has been demonstrated.8,9
Mechanical cleansing of the bowel when possible, decreases the risk of intraoperative bacterial contamination by decreasing the quantity of feces in the intestine while the lumen is opened. Mechanical cleansing, however, does not decrease the concentration of bacteria per gram of feces, only the quantity of feces present. The current veterinary regimen of choice for mechanical bowel cleansing is the technique used for colonoscopy preparation.10,11 The lavage solutions Colyte (Reed & Carnick, Piscataway, NJ) or GoLytely (Braintree Labs, Inc, Braintree, MA) at 80 mg/kg are administered orally in two divided doses four to six hours apart 18 to 24 hours prior to the procedure. These lavage solutions produce an osmotic diarrhea which cleanses the entire gastrointestinal tract. Potential problems with using mechanical cleansing are poor cleansing of the proximal colon when using enemas only, and watery intestinal contents which are more difficult to control once the intestinal tract is open. One human study comparing mechanical preparation alone prior to colorectal surgery demonstrated an over-all postoperative infection rate of up to 45% compared to mechanical preparation with some form of antibiotic solution at 18%.12 To reduce infection rates to an acceptable level after colorectal surgery, some form of antibiotic prophylaxis is also recommended in human colorectal surgery
Systemic antibiotics have been used alone or in combination with mechanical or oral antibiotic bowel preparation for surgical prophylaxis.13,15-17 The rationale for systemic antibiotic prophylaxis is to obtain blood and tissue levels of antibiotic higher than the minimum inhibitory concentration of potential pathogens at the time of maximum tissue contamination. In cases of emergency gastrointestinal surgery, systemic antibiotics are the only feasible method of preoperative prophylaxis.
General recommendations for systemic antibiotic prophylaxis in colorectal surgery include using a drug, or drugs, that are effective against both the aerobic and the anaerobic bacteria found in the large intestine, and that can be administered by a bolus intravenous injection which can rapidly achieve peak serum levels. Bacteriocidal antibiotics with the most narrow effective spectrum, least cost, least toxic side effects, and easiest administration regimen should be used. Drugs should be given preoperatively to obtain effective target-tissue concentrations at the time of potential primary bacterial lodgement; generally they are administered approximately thirty minutes prior to the start of surgery. The pharmacokinetics of the drug should allow it to obtain effective levels against the expected pathogens in the target tissue. Antibiotics should be re-dosed approximately every two half-lives during surgery to maintain effective tissue levels. Finally, prophylactic antibiotics should be discontinued after surgery, with 24 hours being the maximum accepted duration. Continued postoperative antibiotic administration, or administering systemic antibiotics for extended periods prior to surgery, can result in bacterial antibiotic resistance and superinfections.
Systemic antibiotic prophylaxis for colorectal surgery can be broken into combination therapy regimens and monotherapy regimens. The most commonly used combination antibiotic regimens for human colorectal surgery are aminoglycosides, such as gentamicin, kanamycin, amikacin, or tobramycin along with lincomycin, clindamycin, or metronidazole.14,18 Effective monotherapy drugs used for antimicrobial prophylaxis in colorectal surgery include cefoxitin, several third generation cephalosporins, and ampicillin/sulbactam.8,14,19 Cefoxitin has been recommended by several authors as the systemic prophylactic antibiotic of choice for colorectal surgery in veterinary medicine.20-22 The drug is a single agent intravenous antibiotic that has a low toxicity, is relatively inexpensive, and has good bacteriocidal effects against the primary bacterial pathogens. Cefoxitin dosage recommendations in small animals range from 6 to 30 mg/kg IM or IV given every eight hours.21,23 With a half-life of 41 to 59 minutes, cefoxitin should be redosed every 1.5 to 2 hours as a surgical prophylaxis.
The above protocols are predominately based upon research on human colorectal surgery. While controversial, the author only uses first generation cephalosporins as a single agent systemic antibiotic prophylaxes, with no local oral antibiotics or mechanical cleansing. The author has not noted any increase in morbidity or mortality in dogs and cats using this minimal bowel preparation.
Surgical Techniques
Approaches
The colon and rectum can be approached through a ventral midline celiotomy, through a partial or complete pubic (ischialpubic) osteotomy, by a dorsal approach, by a lateral perineal approach, by prolapsing the distal rectal mucosa, or by a rectal pull-through (Figure 20-24).
A caudal ventral midline celiotomy from 2 to 3 cm cranial to the umbilicus extending to the pubic rim permits access to the entire colon and the colorectal junction. The patient should be clipped and aseptically prepared from midthorax to beyond the caudal edge of the pubis. Laterally, the skin preparation should extend slightly beyond the flank folds. The prepuce of male dogs should be flushed with a dilute chlorhexadine or betadine solution.
Exposure to the proximal and middle rectum can be made by extending the caudal midline celiotomy through a partial or complete pubic osteotomy, respectively. The skin incision is extended caudally over the pubis. For a partial pubic osteotomy, the aponeurosis of the gracilis and adductor muscles are incised on the midline and reflected laterally (Figure 20-25A).24 The obturator nerve and vessels lie at the cranial lateral edge of each obturator foramina, and must be protected. Drill holes are made on each side of the osteotomies to facilitate later repair of the defect (Figure 20-25B) and to the drill holes. Guarding the soft tissue, the pubis is then cut on both sides with a sagittal saw, Gigli wire, osteotome, or bone cutter. The cut should be made 2 to 3 mm medial to the lateral edge of each obturator foramina. Leaving the periosteum and soft tissue attached caudally to the floor of the pelvis, a third osteotomy is made joining the caudal edges of the obturator foramina. The pubis is then hinged caudally as a caudally attached flap (Figure 20-25C). The flap is reattached with two orthopedic wires through the pre-drilled holes. Approach to the rectum through a complete pubic osteotomy is performed in a similar manor; however, the caudal osteotomies are made from the obturator foramina transversely through the caudal ischii.25 The ischial-pubic flap is then hinged to one side (Figure 20-25D). Before the osteotomies, drill holes are made on each side of each osteotomy to facilitate repair of the flap. Drill holes craniaocaudally along one side of the pubic symphysis have been recommended to aid in reattachment of the muscle aponeuroses. A urinary catheter is used in male dogs to allow easy identification of the urethra so it can be protected.

Figure 20-24. Approaches to the colon based on the area of interest (Cross hatched area is the middle 1/3 of the rectum). A. Celiotomy for any area of the colon to just cranial to the pubis. B. Pubic osteotomy for any area just cranial to and within the pelvic canal. This approach can be combined with a celiotomy. C. Rectal pull-through for any lesion caudal to the pelvic reflexion. This procedure will likely result in fecal incontinents. D. Dorsal approach for the middle 1/3 of the rectum to just cranial to the anus. E. Lateral approach for one side or the other of the distal middle 1/3 of the rectum to just cranial to the anus. F. Distal rectal mucosal prolapse for lesions of the distal 1/3 of the rectum and anus.

Figure 20-25. Approach to the colon and rectum through a pubic osteotomy. See text for details. A. The aponeurosis of the gracili and adductor muscles are incised on the midline and reflected laterally. Note the obturator nerve and vessels at the cranial lateral edge of the obturator foramina. B. Osteotomy sites and drill holes for a partial pubic osteotomy. C. After reflecting the pubic floor segment caudally, the rectum is visible under the urinary tract. D. Reflecting the pubic floor laterally after a complete osteotomy, the entire ventral rectum can be visualized.
The dorsal approach to the rectum is an easy one that allows good visualization of the middle and caudal rectum, but not the anal canal. The patient is placed in ventral recumbency with the pelvis elevated and the hindlimbs hanging over the back edge of the surgery table (Figure 20-26). The back edge of the table is padded to prevent pressure on the femoral nerves. The tail is fixed over the back with tape. A curvilinear incision is made dorsal to the anus from just above one ischiatic tuberosity to the other. The subcutaneous fat is dissected to the underlying muscles. The thick paired rectococcygeus muscles are identified dorsally, isolated, and transected (Figure 20-27). Depending on the amount of rectum that needs to be resected, circumferential dissection of the rectum can be performed. The levator ani muscles on either side of the rectum can be partially transected to the level of the caudal rectal nerves to aid in the rectal approach. The external anal sphincter can also be elevated caudally. Stay sutures are placed around the area of the rectum to be excised to keep tissue from retracting into the pelvic canal. Stay sutures can also be used to partially rotate the rectum and gain better exposure to the lateral and ventral surfaces. Full circumferential segments of rectum can be resected, or smaller masses, or lacerated tissue, can be resected with an elliptical incision in the rectum (Figure 20-28). The rectum is closed as previously described using sutures or staples. The transected muscle bellies and skin are closed routinely. Rarely, drains may be necessary in contaminated rectal lacerations, however, the drains should not touch the anastomosis as this may predispose the wound to dehiscence.
The lateral perineal approach is rarely indicated to expose one side or the other of the caudal portion of the rectum. The initial approach is identical to that used for repair of a perineal hernia. The rectum is approached by separating the external anal sphincter and the levator ani muscles.
Distal rectal masses that are small and noninvasive can be approached by prolapsing the caudal rectal tissue through the anus. This can be performed digitally or by placing a stay suture or allis tissue forceps oral to the mass (Figure 20-29). Stay sutures are used to retract the rectum while the affected tissue is resected. The rectum is closed in a single layer with a simple interrupted or a continuous suture pattern. The stay sutures are released to allow the rectum to retract into the pelvic canal.
Approaches to the middle and distal thirds of the rectum can be approached through various pull-through techniques. These techniques can involve prolapsing tissue, extensive tissue dissection, or a combination of the two.
Resection and Anastomosis
Resection and anastomosis of the colon are performed in a manner similar to that of the small intestine. After making an approach to the affected segment of colon, a complete exploration of the area is performed. To determine the extent of the disease process, regional lymph nodes and adjacent organs are carefully examined at surgery. Examination for unrelated, but potentially complicating disease processes should be performed during celiotomy approaches.
The intestinal segment to be resected should be carefully isolated with laparotomy sponges moistened with warm isotonic saline (Figure 20-30A). The exposed tissue should be kept moist at all times to prevent desiccation and trauma. Two to three layers of laparotomy sponges or 4x4 sponges allows for removal of contaminated material with minimal chance for further contamination. Contaminated material should be removed from the sterile field as soon as possible to prevent further spread of contamination. An area for contaminated surgical instruments on the sterile field can be made with a dry lap sponge or drape. As soon as the instruments are no longer needed, they should be removed from the instrument table.
Carmalt forceps can be placed at the edges of the colonic segment to be resected. A minimum of 1 to 2 cm of healthy vascularized tissue should be included within the segment to be resected. Carmalt forceps can be placed perpendicularly across the colon, or they can be placed to back cut on the antimesenteric side, creating a larger anastomotic diameter. Atraumatic clamps (Doyen forceps, vascular forceps, bobby pins, or an assistant’s finger tips) are placed 4 to 5 cm to the outside of the Carmalt forceps. The atraumatic forceps keep luminal contents from leaking from the cut ends of the colon, as well as assisting in manipulation of the cut ends of the colon. Any remaining mesocolon is then resected as far from any vessels as possible. The affected colon segment can then be resected with a scalpel, using the outside edge of the Carmalt forceps as a guide. Colonic mucosa commonly everts over the cut edge of the intestine. It is easier to anastomose the colon if the mucosa is resected level to the cut edge of the outer colonic wall. This procedure is easily performed using Metzenbaum scissors. The colonic segments can then anastomosed using a variety of techniques listed below.
After performing and pressure leak testing the colonic anastomosis, the anastomotic site is flushed with saline. Layers of laparotomy sponges can be removed in between flushing the anastomosis. Surgical gloves, instruments, and other contaminated equipment should be changed at this time. A sterile fenestrated drape can be placed over the surgery site. If there is no obvious contamination of the abdomen, abdominal lavage is not necessary. Otherwise, the abdomen should be lavaged with warm isotonic saline until the effluent is clear. The mesocolon should be closed with a continuous suture pattern of 3-0 or 4-0 absorbable material. Care should be taken so as not to damage the adjacent blood supply to the colon. The surgical approach is then closed in a routine manor.

Figure 20-26. Patient positioning for a dorsal approach to the rectum (See text for details). The curved dotted line indicates the location of the incision.

Figure 20-27. The taught thick paired rectococcygeus muscles (under forceps) are easily identified after the incision is made and subcutaneous fat is dissected. The muscles can be cut anywhere along the belly (dotted line) and distracted with stay sutures.

Figure 20-28. After cutting and retracting the rectococcygeus muscles the levator ani muscles on either side of the rectum can also be partially transected to expose the rectal lesion. The rectum can be resected with an elliptical incision or circumferentially as needed to remove the lesion (dotted lines). Make certain to use sufficient stay sutures in normal rectal tissue to keep the cut edges from retracting away from the surgery site.

Figure 20-29. Prolapsing caudal rectal mucosa can be done by grasping the mucosa oral to the lesion with atraumatic forceps or stay sutures. Sufficient full thickness stay sutures in normal rectum should be placed to keep the cut edges well defined and prolapsed until the defect is closed.

Figure 20-30. Preparation for colonic resection and anastomosis. A. Moistened laparotomy sponges are placed under the balfour retractor and wrapped around the base of the mesentery to isolate the affected colonic segment. B. The blood supply to the affected segment is double ligated. For short colonic segments, individual vasa recta should be ligated, preserving the longitudinal mesenteric vessels. For longer colonic segments, the longitudinal mesenteric vessels can be ligated. Carmalt forceps are placed oral and aboral to the segment of colon to be resected, making certain to include all of the avascular bowel. Atraumatic forceps are then placed outside the carmalt forceps. The affected colonic segment is now transected using the outside of the carmalt forceps as a guide.
Methods of Colonic Anastomosis
After intestinal resection, the continuity of the intestinal tract can be reconstructed using three basic anastomotic techniques: end-to-end, side-to-side, and end-to-side. When hand suturing is used, the end-to-end intestinal anastomosis is the easiest and quickest technique to perform and results in a more physiologic reconstruction. Side-to-side and end-to-side anastomosis of the intestine have also been incriminated with formation of blind pouches where bacterial overgrowth and resulting malabsorption can occur.
When a disparity of luminal diameters is present, especially as seen with ileocolic anastomoses, several techniques are available to aid in end-to-end anastomoses. A funneled closure is the simplest anastomosis if minor disparities of luminal diameters exist. Sutures are placed equidistant around the circumference of the lumen ends. This results in stretching of the smaller luminal opening and constricts the larger luminal opening (Figure 20-31A). With larger luminal disparities, the smaller diameter intestine can be cut at an angle, with more tissue removed from the antimesenteric border (Figure 20-31B). If a luminal disparity still exists, the antimesenteric border of the smaller-diameter intestine can be further incised 1 to 2 cm. Two triangular flaps of intestinal wall can then be cut off each side of the incision, leaving an ovoid stoma that can be anastomosed to the larger-diameter intestine (Figure 20-31C). Finally, if the smaller-diameter intestinal lumen cannot be opened widely enough, the larger-diameter intestine can be partially sutured closed until the luminal diameters are equal (Figure 20-31D).
Two-layer anastomotic closures of the colon are no longer advocated. Several studies have demonstrated there is no increase in intestinal dehiscence and actually an increased healing rate, using a single-layer closure versusa two-layer closure.26,27 In fact, two-layer anastomotic closures have been demonstrated to have significantly greater incidences of dehiscence and stricture formation in the rectum because of avascular necrosis of the tissue incorporated in the inner suture pattern.26 Leakage at the anastomosis site is not a problem if the omentum is healthy and intact and the patient is not hypoproteinemic. A fibrin seal will form at the anastomosis site within about 3 hours in most patients.28
Numerous intestinal anastomosis studies have been performed comparing simple continuous, simple interrupted, inverting, everting, or appositional suture techniques. The anastomosis techniques that are the easiest to perform, with the least leakage, the least adhesion formation, and the best histologic healing, have been the single-layer simple interrupted approximating techniques. In 1968 Poth and Gold described the crushing appositional anastomosis technique in human patients.29 This technique involved a through-and-through suture, which was then tightened to cut through all the layers of the intestine except the tough submucosa (Figure 20-32A). This technique kept the suture from being exposed to the luminal surface, where it could become infected, and from exposure to the abdominal lumen and serosal surface, where adhesions could form. At about the same time the crushing technique was developed, DeHoff investigated the use of a simple interrupted approximating technique for intestinal anastomosis in dogs (Figure 20-32B).30 Both appositional techniques maintain luminal diameter, diminish adhesion formation, and allow for rapid primary healing of the intestinal anastomosis. Some eversion commonly occurs with both these appositional techniques, resulting in adhesions and some altered healing.28 The Gambee suture pattern helps eliminate the slight eversion caused by the simple appositional suture patterns (Figure 20-32C).
Various suture materials are used successfully for intestinal anastomosis, including monofilament and braided sutures of absorbable and nonabsorbable sutures. The monofilament absorbable sutures polydioxanone, polyglyconate, and poliglecaprone 25 are closest to the ideal suture material available for intestinal anastomosis today. Nonabsorbable monofilament suture material such as nylon or polypropylene may be useful in patients that are expected to have delayed tissue healing. The braided absorbable sutures polyglycolic acid and polyglactin 910 are absorbed in a relatively short period of time. These sutures have a constant absorption rate which is not affected by infection, so infected suture tracts and granulomas are of little concern. The biggest problem with the absorbable braided suture is the tissue trauma (drag or chatter) as they are pulled through tissue. The surface characteristics of braided sutures have been shown by electron microscopy to increase trauma to the tissue they have been pulled through, as opposed to smooth surfaced monofilament suture material.31 In small animal colonic surgery, a size 3-0 to 4-0 suture should have sufficient tensile strength to hold intestinal tissue.
A swaged-on reverse cutting or taper-cut suture needle is recommended for colonic surgery. These suture needles facilitate penetration of the intestine’s tough submucosa with the least effort and tissue trauma. Taper-point or narrow-taper needles have been suggested by some surgeons, because less intestinal leakage occurs around the suture tract. The increased trauma of passing the taper needle through the submucosa must be balanced with this minor benefit.
The number of sutures placed to form an anastomosis should be the minimum needed to prevent leakage of the anastomosis. Most intestinal anastomosis techniques describe placing sutures 2 to 4 mm from the cut serosal surface and 3 to 4 mm apart. This averages to approximately 12 to 16 simple interrupted sutures evenly spaced around the anastomosis. The first suture s normally placed at the mesenteric border because this is the most difficult to see, and this area has the highest incidence of leakage and dehiscence (Figure 20-33A). The second suture is normally placed at the antimesenteric border, with the remaining sutures filling in the area between the first two sutures (Figure 20-33B). The anastomosis can be tested by filling the segment of intestine with saline under slight pressure, or milking luminal contents across the anastomosis and looking for leaks. Any anastomosis will leak if too much pressure is applied. Too many sutures decrease anastomosis healing by interfering with blood supply to the intestinal edges. Some authors recommend wrapping or even suturing the omentum around the anastomosis site. This is normally not necessary because the omentum naturally moves to cover any leaks in an intestinal anastomosis.
Surgical stapling is another method of intestinal anastomosis that has become increasingly popular. The device commonly used in colonic resection and anastomosis is the circular stapler manufactured by Ethicon (Proximate ILS, Ethicon, Inc., Somerville, NJ) or United States Surgical (CEEA, United States Surgical Corp., Norwalk, CT). The circular stapler inverts the intestinal ends and places two circumferential rows of staggered B-shaped sutures. The device then cuts out a donut-shaped section of the inverted tissue from the ends of the intestine being joined. The circular stapler can be inserted through the anus or through an access incision in the intestine. A modified Furness clamp, or purse string stapler, is used to place a purse string suture around the ends of the intestinal segments to be joined. One intestinal end is then slipped over the cartridge end, and the other intestinal segment is placed over the anvil. The purse string sutures are then tied to the movable central shaft between the cartridge head and anvil. The shaft is shortened, compressing the cartridge to the anvil with the intestinal ends in between. The stapler is then fired, forming the anastomosis and cutting out the purse string along with the tissue in the middle of the lumen (Figure 20-34). The circular stapler forms a true inverting anastomosis. Occasionally, the result is the same problem caused by a hand-sutured inverting anastomosis, that is, luminal strictures. The circular stapler is a technically demanding stapler to use. Improper usage of the stapler, or poor surgical technique, may result in anastomotic stricture or dehiscence. When performed by an experienced surgeon, the stapled anastomosis line has been demonstrated to leak less, to be better aligned, and to heal better than single-layer hand-sutured anastomoses.32 Ordinary skin staplers have also been found to provide safe anastomoses.33 Skin staplers are especially helpful in repairing multiple intestinal perforation caused by gunshot wounds. Various other sutureless intestinal anastomosis techniques have been studied through the years, from cyanoacrylate adhesives and fibrin glue, to laser welding and non-absorbable and absorbable anastomosis rings. For various reasons anastomosis techniques other than sutures and staples have not met with wide acceptance.

Figure 20-31. Anastomosis of dissimilar sized lumens. See text for details. A. Funneled closure. B. Oblique transection of the smaller lumen. C. Spatulated closure. D. Partial over-sew.

Figure 20-32. A. The simple interrupted appositional suture pattern. B. The Poth and Gold crushing pattern. Notice the suture crushes through all of the tissue layers to hold just the submucosa C. The Gambee suture pattern. Notice the suture passes through the mucosa and causes very slight tissue inversion.

Figure 20-33. Technique for colonic anastomosis. A. The two colonic segments are held together with the aid of the atraumatic forceps as a mesenteric and then antimesenteric suture is placed to start the anastomosis. These first 2 sutures can be used as stay sutures to handle the bowel. B. The anastomosis is completed with a single layer appositional suture pattern filling in the sutures between the stay sutures. The mesentery is closed with a simple continuous suture pattern.
Colonic Healing
The colon follows the same stages of healing as skin and other soft tissue: inflammation, debridement, repair, and maturation.34 A unique property of colonic healing, however, involves the balance of collagen synthesis and degradation. During the first 3 to 5 days after wounding, collagen synthesis is competing with collagenolysis.34-36 This is important, because the collagen content of a wound has been directly correlated with wound strength.36 There is an especially high turnover rate of collagen in the wounded colon.34-37 Earlier work suggests that as much as 40% of the rat colon’s original collagen content, throughout the entire colon, is lost to collagenolysis during the first 4 to 6 days after wounding.37 However, early studies have over emphasized the drop in collagen content in colonic wounds. With the use of more advanced techniques in measuring the collagen content of a wound, researchers have found that the drop in collagen content is not as dramatic as originally thought.38 Rapid gain occurs in colonic tensile strength between the third and seventh days after wounding.38 Local factors in the colon can, however, shift a wound towards increased collagen lysis. Traumatic handling of colonic tissue, bacterial contamination, foreign material, and certain suture patterns used for intestinal anastomoses all increase the amount of collagenase produced locally in colonic tissue.
Biopsy
Full-thickness biopsy techniques of the colon are performed similar to those in the small intestine. Luminal contents are milked from the biopsy site, and the site is isolated with a moistened laparotomy sponge. A full-thickness longitudinal incision approximately 1 to 2 cm long is made in the antimesenteric colonic wall. A full-thickness segment approximately 2 to 3 mm wide is cut from the side of the incision. Care should be taken not to crush the sample with forceps. The colonic defect is then closed transversely using simple interrupted sutures (Figure 20-35). Large diameter round dermal punches have also been successfully used for full thickness biopsies. Care must be taken to only cut through one side of the intestine.

Figure 20-34. Distal colorectal anastomosis with a circular stapler. See text for details. A. A modified Furness clamp is used to place a purse string suture on the aboral intestinal segment (top of figure). The affected orad segment is isolated and resected. B. A transrectal circular stapler is placed to the level of the aboral purse string suture. The purse string is then tied around the center anvil of the stapler. C. A purse string suture around the orad intestinal segment is used to secure the segment to the circular stapler anvil cranial to the aboral segment. D. The circular stapler is then compressed and fired to form the anastomosis.
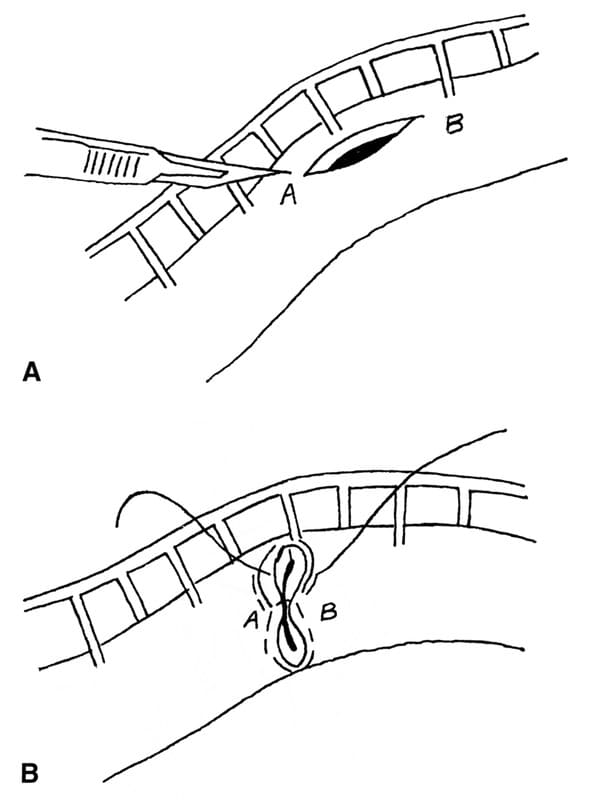
Figure 20-35. A. Longitudinal incisions in bowel can be closed transversely B. to prevent reduction of the luminal diameter.
Postoperative Care and Complications
Immediate Postoperative Care
Patients undergoing major colorectal surgery often require significant postoperative care. Intravenous fluids should be continued postoperatively until the patient is taking food and water by mouth and the patient’s temperature is below approximately 103.6°F. Rectal thermometers should be used with care. Infrared ear thermometers are preferred in animals that have undergone rectal surgery. Patients should be observed for signs of peritonitis for the first 3 to 5 days after surgery. These signs including fever, depression, anorexia, abdominal pain, vomiting, and shock. Postoperative antibiotics are generally not recommended unless intraoperative signs of established infection are present. Inappropriate use of antibiotics can mask signs of peritonitis and can result in superinfections. The author routinely administers injectable narcotics immediately after endotracheal extubation, with a pain protocol for subsequent doses as needed. Oral or transdermal narcotics and anti-inflamatories are dispensed for 3 to 5 days after surgery. Clinically, patients appear to be comfortable within 12 to 24 hours after surgery. Patients can be offered water once they are fully awake from anesthesia. A low-residue diet can be offered within 12 to 24 hours after surgery. This diet should be continued for the first 2 to 3 weeks, after which the animal’s normal diet can be gradually introduced. Stool consistency, color, and presence of blood should be carefully monitored. The patient’s first bowel movement commonly contains a large amount of soft to liquid, dark stool whith whole blood. Stool softeners can be administered as necessary to maintain a semifirm consistency. Patients that recover without complications are usually discharged on the second or third postoperative day.
Early Complications
The most serious early postoperative complications of colorectal surgery are infection and fecal incontinence. In a review of intestinal surgery in dogs and cats, patients with peritonitis had a morality rate of 31%.39 Infection after colorectal surgery can result from preoperative trauma, interoperative contamination of the abdomen, and intestinal dehiscence. While rare, rectal perforations caused by pelvic fractures can be successfully treated if diagnosed before significant contamination of surrounding tissue occurs. In one small study of patients with rectal perforations caused by pelvic fractures, definitive surgical treatment performed within 24 hours of occurrence resulted in no mortality.5 All patients with delayed diagnosis or treatment had fatal outcomes. Postoperative intestinal dehiscence is one of the most common causes of infection. In one study, dehiscence resulted in a mortality rate of 80%.39 This same study found no significant difference between small and large intestinal dehiscence rates with an average of 7%. Many factors can result in colorectal dehiscence, including poor surgical technique, traumatic tissue handling, disrupted blood supply, poor suture placement, tension on the anastomosis, improper use of drains, delayed healing, and inappropriate postoperative care. Discriminant analysis in one study demonstrated a sensitivity of 91% with a specificity of 83% using a model where dogs having 2 or more risk factors (preoperative peritonitis, serum albumin concentrations less than or equal to 2.5 g/dL, and intestinal foreign bodies) resulted in intestinal anastomotic leakage.40
The clinical signs of peritonitis have been described previously. Diagnostic procedures for postoperative peritonitis and dehiscence may include abdominocentesis or peritoneal lavage, gentle rectal palpation, complete blood count, plain abdominal and pelvic radiographs, and abdominal ultrasonography. Contrast radiographs or proctoscopy are contraindicated and may result in further abdominal contamination.
If signs of peritonitis or intestinal dehiscence are present, the animal should be supported with appropriate intravenous fluids and started on an appropriate therapeutic regimen of antibiotics based on culture and sensitivity testing. The surgeon should not hesitate to perform a “second-look operation” if indicated. Patients with peritonitis do not generally stabilize without adequate abdominal drainage and, if necessary, repair of leaking intestine. Open abdominal drainage is one successful method of surgical drainage that also allows serial evaluation of the affected colorectal segment. For recurrent dehiscence, or areas of questionable vascularity, the use of omental flaps, jejunal patch grafts, and peritoneal muscle flaps have been reported in the veterinary literature. The use of diversional colostomies have been reported in the human and equine literature. This technique has been reported in dogs, and may be a viable treatment option in selected small animal cases.41,42
Fecal incontinence, while not in itself fatal, often results in euthanasia of house pets. Fecal incontinence can be divided into reservoir and sphincter incontinence.43 Patients with reservoir incontinence generally have a conscious, but frequent, need to defecate. This condition is in contrast to unconscious anal dribbling of feces found in patients with sphincter incontinence. Reservoir incontinence can be caused by colorectal irritability, decreased rectal capacity or compliance, increased propulsive motility, and increased fecal volume. One author suggests that fecal continence will be retained if less than 4 cm of rectum is resected, or greater than 1.5 cm of distal rectum is retained in the dog.44 Treatment for surgically induced reservoir incontinence includes anti-inflammatory drugs, drugs that slow intestinal transit time, dietary manipulation to decrease fecal volume, and surgical techniques that increase rectal capacity. Some animals, over time, may develop ileoanal continence. This is where the ileum distends, taking over the reservoir function of the colon and rectum. The causes of sphincter incontinence are not fully understood, but they include neurologic and muscular trauma or disease. Along with the external anal sphincter, studies have demonstrated muscles of the pelvic girdle, especially the levator ani, play an important role in fecal continence. Treatment for surgically induced sphincter incontinence may include the same medical treatments used for reservoir incontinence. Surgical treatments for sphincter incontinence include reconstruction of the pelvic girdle and external anal sphincter, sphincteroplasty, replacing muscles of continence with muscle flaps or synthetic material, and ileal J-pouch anal anastomoses.
Late Complications
The most common late complication of colorectal surgery is lumenal stricture. Most intestinal anastomoses result in some degree of lumenal stricture. Single-layer and double-layer inverting suture patterns have been reported to result in 39% and 54% lumenal stricture respectively. This was compared with 4% lumenal stricture using an approximating Gambee pattern closure.45 In another study, colonic anastomoses created with a 25 mm circular stapler where found to result in an average 32% decrease in lumenal diameter at the anastomosis.46 Too much tissue inversion, suture patterns that restrict the luminal diameter, tension at the anastomosis, and extra lumenal adhesions can result in excessive lumenal stricture. Diagnosis of colorectal stricture include clinical signs, rectal or abdominal palpation, contrast radiographs, abdominal ultrasound, and proctoscopy. Most commonly, colorectal strictures are treated medically with diet change and stool softeners. Treatment of severe colorectal strictures may require resection and anastomosis of the strictured segment, or, less commonly, mechanical dilation may be attempted. Mechanical dilation can be achieved digitally, by bougienage, or with balloon catheters. Care should be taken not to perforate the intestinal lumen using dilation techniques.
A less common long term complication is associated with the use of nonabsorbable suture material when it is used in a continuous suture pattern.47 The nonabsorbable suture can be extruded partially into the intestinal lumen where foreign bodies have been reported to attach causing intestinal obstructions. The use of absorbable suture is recommended when making intestinal anastomoses with a continuous suture pattern.
References
- Taylor, W.T., Weber, R.J.: Functional Mammalian Anatomy (with special reference to the cat). Toronto, D. Van Nostrand Co., Inc., 1951.
- Evans, H.E., Christensen, G.C.: Miller’s Anatomy of the Dog. 2nd Ed. Philadelphia, W.B. Saunders Co., 1979.
- Schaller, O., Constantinescue, G.M.: Illustrated Veterinary Anatomical Nomenclature. 1992.
- Goldsmid, S.E., Bellenger, C.R., Hopwood, P.R., et al: Colorectal blood supply in dogs. Am. J. Vet. Res., 54:1948-1953, 1993.
- Lewis, D.D., Beale, B.S., Pechman, R.D., et al: Rectal perforations associated with pelvic fractures and sacroiliac fracture-separations in four dogs. J. Am. Anim. Hosp. Assoc., 28:175-181, 1992.
- Valerius, K.D., et al: Adenomatous polyps and carcinoma in situ of the canine colon and rectum: 34 cases (1982-1994). J. Am. Anim. Hosp. Assoc., 33:156, 1997.
- Greene, C.E.: Infectious Diseases of the Dog and Cat. Philadelphia, W.B. Saunders Co., 1990.
- Dow, S.W.: Management of anaerobic infections. Vet. Clin. N. Am. S.A. Pract., 18:1167-1182, 1988.
- Boothe, D.M.: Anaerobic infections in small animals. Prob. Vet. Med., 2:330-347, 1990.
- Richter, K.P., Cleveland, M.vB.: Comparison of an orally administered gastrointestinal lavage solution with traditional enema administration as preparation for colonoscopy in dogs. J. Am. Vet. Med. Assoc., 195:17271731, 1989.
- Burrows, C.F.: Evaluation of a colonic lavage solution to prepare the colon of the dog for colonoscopy. J. Am. Vet. Med. Assoc., 195:1719- 1721, 1989.
- Peck, J.J., Fuchs, P.C., Gustafson, M.E.: Antimicrobial prophylaxis in elective colon surgery: Experience of 1,035 operations in a community hospital. Am. J. Surg., 147:633-637, 1984.
- Penwick, R.C.: Perioperative antimicrobial chemoprophylaxis in gastrointestinal surgery. J. Am. Anim. Hosp. Assoc., 24:133-145, 1988.
- Burnakis, T.G.: Surgical antimicrobial prophylaxis: Principles and guidelines. Pharmacotherapy., 4:248-271, 1984.
- Condon, R.E., Bartlett, J.G., Greenlee, H., et al.: Efficacy of oral and systemic antibiotic prophylaxis in colorectal operations. Arch. Surg., 118:496-502, 1983.
- Washington, II J.A., Dearing, W.H., Judd, E.S., et al.: Effect of preoperative antibiotic regimen on development of infection after intestinal surgery: Prospective, randomized, double-blind study. Ann. Surg., 180:567-572, 1974.
- Baum, M.L., Anish, D.S., Chalmers, T.C., et al.: A survey of clinical trials of antibiotic prophylaxis in colon surgery: Evidence against further use of no-treatment controls. N. Eng. J. Med., 305:795-799, 1981.
- Onderdonk, A.B., Bartlett, J.G., Louie, T., et al.: Microbial synergy in experimental intraabdominal abscess. Infect. Immun., 13:22-26, 1976.
- De La Hunt, M.N., Karran, S.J., Chir, M.: Sulbactam/ampicillin compared with cefoxitin for chemoprophylaxis in elective colorectal surgery. Dis. Colon Rectum, 29:157-159, 1986.
- Bright, R.M.: Treatment of feline colonic obstruction (megacolon). In: Current Techniques in Small Animal Surgery, 3rd Ed.. Edited by M.J. Bojrab. Philadelphia, Lea & Febiger, 263-265, 1990.
- Rosin, E., Dow, S., Daly, W.R., et al.: Surgical wound infection and use of antibiotics. In: Textbook of Small Animal Surgery, 2nd Ed.. Edited by D. Slatter. Philadelphia, W.B. Saunders Co.84-95, 1993.
- Huss, B.T., Payne, J.T., Wagner-Mann, C.C., et al.: Pharmacokinetic disposition of cefoxitin in serum and tissue during colorectal surgery in cats. In preparation, 1996.
- Plumb, D.C.: Veterinary Drug Handbook, 3rd Ed.. White Bear Lake, PharmaVet Pub 117-118, 1995.
- Walshaw, R.: Removal of rectoanal neoplasms. In: Current Techniques in Small Animal Surgery, 3rd Ed. Edited by M.J. Bojrab. Philadelphia, Lea & Febiger, 274-290, 1990.
- Allen, S.W., Crowell, W.A.: Ventral Approach to the pelvic canal in the female dog. Vet. Surg., 20:118-121, 1991.
- Everett, W.G.: A comparison of one layer and two layer techniques for colorectal anastomosis. Br. J. Surg., 62:135-140, 1975.
- Ballantyne, G.H.: The experimental basis of intestinal suturing: Effect of surgical technique, inflammation, and infection on enteric wound healing. Dis. Colon Rectum, 27:61-71, 1984.
- Ellison, G.W.: End-to-end anastomosis in the dog: A comparison of techniques. Compend. Contin. Ed. Pract. Vet., 3:486-494, 1981.
- Poth, E.J., Gold, D.: Intestinal anastomosis: A unique technic. Am. J. Surg., 116:643-647, 1968.
- DeHoff, W.D., Nelson, W., Lumb, W.V.: Simple interrupted approximating technique for intestinal anastomosis. J. Am. Anim. Hosp. Assoc., 9:483-489, 1973.
- Lord, M.G., Broughton, A.C., Williams, H.T.G.: A morphologic study on the effect of suturing the submucosa of the large intestine. Surg. Gynecol. Obstet., 146:211-216, 1978.
- Stoloff, D., Snider, III T.G., Crawford, M.P., et al.: End-to-end colonic anastomosis: A comparison of techniques in normal dogs. Vet. Surg., 13:76-82, 1984.
- Coolman, B.R., Erhart, N., Pijanowsk, G., et al: Comparison of skin staples with sutures for anastomosis of the small intestine in dogs. Vet. Surg., 29:293-302, 2000.
- Ravo, B.: Colorectal anastomotic healing and intracolonic bypass procedure. Surg. Clin. N. Am. S. A., 68:12671294, 1988.
- Ellison, G.W.: Wound healing in the gastrointestinal tract. Sem. Vet. Med. Surg. S.A., 4:287-293, 1989.
- Ballantyne, G.H.: Intestinal suturing: Review of the experimental foundations for traditional doctrines. Dis. Colon Rectum, 26:836-843, 1983.
- Cronin, K., Jackson, D.S., Dunphy, J.E.: Changing bursting strength and collagen content of the healing colon. Surg. Gynecol. Obstet., 126:747-753, 1968.
- Irvin, T.T., Hunt, T.K.: Reappraisal of the healing process of anastomosis of the colon. Surg. Gynecol. Obstet., 138:741-746, 1974.
- Wylie, K.B., Hosgood, G.: Mortality and morbidity of small and large intestinal surgery in dogs and cats: 74 cases (1980-1992). J. Am. Anim. Hosp. Assoc., 30:469-474, 1994.
- Ralphs, S.C., Jessen, C.R., Lipowitz, A.J.: Risk factors for leakage following intestinal anastomosis in dogs and cats: 115 cases (1991- 2000). J. Am. Vet. Med. Assoc., 223:73-77, 2003.
- Swalec-Tobias, K.M.: Rectal perforation, rectocutaneous fistula formation, and enterocutaneous fistula formation after pelvic trauma in a dog. J. Am. Vet. Med. Assoc., 205:1292-1296, 1994.
- Chandler, J.C., Kudnig, S.T., Monnet, E.: Use of laparoscopic-assisted jejunostomy for fecal diversion in the management of a rectocutaneous fistula in a dog. J. Am. Vet. Med. Assoc., 226:746-751, 2005.
- Guilford, W.G.: Fecal incontinence in dogs and cats. Compend. Contin. Ed. Pract. Vet., 12:313-326, 1990.
- Holt, D., Johnston, D.E., Orsher, R., et al.: Clinical use of a dorsal surgical approachto the rectum. Compend. Contin. Ed. Pract. Vet., 13:1519-1528, 1991.
- Hamilton, J.E.: Reappraisal of open intestinal anastomoses, Ann. Surg., 165:917, 1967.
- Yamane, T., Takahashi, T., Okuzumi, J., et al.: Anastomotic stricture with the EEA stapler after colorectal operation in the dog. Surg. Gynecol. Obstet., 174:41-45, 1992.
- Milovancev, M., Weisman, D.L., Palmisano, M.P.: Foreign body attachment to polypropylene suture material extruded into the sm all intestinal lumen after enteric closure in three dogs. J. Am. Vet. Med. Assoc., 225:17131715, 2004.
Management of Rectal Prolapse
Mark H. Engen
Although rectal prolapse can occur with any condition that causes prolonged tenesmus, it is most common in heavily parasitized animals that have severe diarrhea and tenesmus. Other causes of straining resulting in rectal prolapse are dystocia, urolithiasis, intestinal neoplasms and foreign bodies, prostatic disease, perineal hernia, constipation congenital defects, and postoperative tenesmus after anal or perineal surgery.
Diagnosis
The diagnosis of rectal prolapse is made by visual observation of a tubelike mass, of varying length, protruding from the anus. If rectal prolapse is diagnosed early, the protruding tissue may be short, and the prolapsed mucosa will appear bright red and nonulcerated. In patients with rectal prolapse of long duration, the protrusion is longer, and the mucosa appears red or black and is either ulcerated or necrotic.
True rectal prolapse must be differentiated from prolapsed intussusception of the intestine or colon. These conditions can be differentiated by passing a probe between the anus and the prolapsed mass. The probe can be passed if an intussusception is present, but it cannot be passed if a rectal prolapse has occurred. To achieve a permanent cure for rectal prolapse, the underlying cause of tenesmus must be diagnosed and treated.
Nonsurgical Treatment
Treatment to correct a rectal prolapse depends on the viability of the exposed tissue and the size of the prolapse. A small prolapse with viable-appearing mucosa usually can be replaced by using a finger or bougie to reposition the bowel. Topical application of hypertonic sugar solution for 20 to 30 minutes may be helpful in relieving edema, so the prolapse can be reduced more easily. When the prolapse has been reduced, an anal pursestring suture is used to prevent recurrence. General anesthesia or epidural analgesia is used in some patients to facilitate reduction of the prolapse and placement of the anal pursestring suture (Figure 20-36).
After reduction of the prolapse, epidural analgesia prevents straining for several hours. Periodic rectal application of a local anesthetic ointment (1% dibucaine [Nupercainal ointment, Ciba Pharmaceutical, Ciba-Geigy, Summit, NJ]) may be done initially and after removal of the anal pursestring suture to prevent further straining. The anal pursestring suture is left in place for a minimum of 24 to 48 hours, and the animal is given only fluids orally during this time.

Figure 20-36. Placement of anal pursestring suture after reduction of rectal prolapse by manipulation.
Surgical Treatment
When a rectal prolapse cannot be reduced by manipulation and the lack of tissue viability contraindicates reduction, rectal resection and anastomosis are performed. This procedure is performed under general anesthesia or epidural analgesia. The patient is positioned and draped (Figure 20-37A and B). A test tube or a saline-soaked sponge is placed into the lumen of the bowel to prevent fecal contamination. Three stay sutures are placed through the full thickness of both layers of the prolapse to form a triangle (Figure 20-37C and D). The prolapse is then resected 1 to 2 cm from the anus. The anastomosis is performed with a single-layer closure using a simple interrupted suture pattern (Figure 20-37E). Synthetic absorbable suture (3-0 or 4-0) is preferred. The sutures are placed through the full thickness of the incised ends of the bowel. The sutures must pass through the submucosa to ensure proper holding strength. The stay sutures are then removed, and the anastomosis is reduced manually inside the anus.
When the rectal prolapse cannot be reduced by external manipulation, but the rectal tissue is still viable, a celiotomy is performed, and the prolapse is manually reduced by gentle traction on the colon (Figure 30-38A). A colopexy is performed after reduction of the prolapse to prevent recurrence using synthetic absorbable suture (2-0 or 3-0) (Figure 30-38B and C). A colopexy may also be performed in cases of recurrent rectal prolapse that can be reduced by external manipulation. Such a colopexy is rarely needed, however, if the cause of straining has been diagnosed and eliminated.

Figure 20-37. Rectal resection and anastomosis to correct prolapse. A. Positioning of patient on a perineal stand. B. Sterile draping of the prolapse. C. Insertion of test tube into rectum and placement of stay sutures. D. Excision of the prolapsed mass. E. Full-thickness anastomosis of the rectal lumen.

Figure 20-38. Celiotomy and colopexy for treatment of rectal prolapse. A. Abdominal incision and digital replacement of the prolapsed tissue. B. Placement of colopexy mattress sutures. C. Six to eight mattress sutures are placed to complete the colopexy.
Postoperative Care
Topical anesthetic (1% dibucaine) ointment is instilled rectally after correction of any rectal prolapse to prevent further tenesmus. The patient may be fed on the day after the operation. A diet of soft food and a fecal softener (dioctyl sodium sulfosuccinate) also may be administered for 1 week postoperatively. Diarrhea should be treated with neomycin, intestinal coating agents, and anticholinergic drugs. Feces should be examined, and antihelminthic agents should be administered, based on results of fecal examinations for parasitic ova.
In conclusion, once a rectal prolapse has been corrected by surgical or nonsurgical means, recurrence is rare if the cause of the tenesmus has been diagnosed and resolved (e.g., removal of intestinal parasites by worming).
Suggested Readings
Aronson L. Rectum and anus. In: Slatter D, ed. Textbook of small animal Surgery. Philadelphia: Saunders, 2003.
Popovitch CA, Holt D, Bright R. Colopexy as a teatment for rectal prolapse in dogs and cats; a retrospective study of 14 cases. Vet Surg 1994:23:115.
Anal Sac Disease and Removal
Roy F. Barnes and Sandra Manfra Marretta
Introduction
Anal sac disease occurs with an approximate incidence of 12% in the canine, with small breeds being overrepresented compared to large breeds. Cats are infrequently afflicted with anal sac disorders. Conditions which require anal sacculectomy include relief from chronic and recurrent episodes of anal sac impaction or infection, adjunctive treatment of perianal fistulas and local treatment of apocrine gland anal sac adenocarcinoma and other malignant neoplasms.
Anatomy
The anal sacs are cutaneous diverticula ventral and lateral to the anus, between the internal and external anal sphincters. Anal sacs of the dog and cat are composed of large coiled apocrine glands. In addition, the feline anal sac includes a complex series of sebaceous glands. Despite the species difference, all glands will coalesce to form a sac and from the sac, a keratinized epithelial duct arises to carry material to the external environment. In the dog, the anal ducts open into the inner cutaneous zone of the anus while in the cat, the ducts open into a pyramidal prominence 2.5 mm lateral to the anus. Regardless of the duct opening, the anal sacs are typically located at the 4 and 8 o’clock positions with reference to the anus proper.
The blood supply to the anal sac arises from the caudal hemorrodial, perineal and caudal gluteal arteries and veins. The innervation to the anal sac and external anal sphincter is via the pudendal nerve.
Preoperative Care
Prior to anal sacculectomy, patients with anal sac impaction or infection should undergo medical therapy. Failure to resolve any inflammation associated with the anal sac disease prior to surgery may increase the potential for postoperative complications.
Medical therapy consists of expression of the anal sacs and the concurrent instillation of an oil-based antibiotic and corticosteroid-containing ointment into the anal sac. Broad spectrum antibiotics are utilized in animals with severe infection or abscessation of the anal sac. Warm compresses and hydrotherapy are applied to the perineum to improve lymphatic drainage, and cleanse the region. All animals with a suspected neoplasm of the anal sac should undergo appropriate clinical staging with emphasis on determining the potential for local and distant metastasis. Hypercalcemia, if present, should be treated with appropriate medical therapy prior to the induction of general anesthesia. Consideration should also be given to the possibility of postoperative hypocalcemia.
Surgical Technique
Several surgical techniques have been described in the veterinary literature for removal of the anal sacs. Surgeries are divided into open, modified open or closed techniques. The major difference between the techniques is whether or not the anal sac and its duct are incised, thus exposing the lumen. The closed technique is described here. It is imperative that a closed anal sacculectomy be performed for the treatment of apocrine gland anal sac adenocarcinoma or any other malignancy of the anal sac or duct.
Regardless of which surgical technique is performed, the protocol for surgical preparation is similar. The diseased anal sac is expressed to expel its contents with subsequent instillation of a dilute antiseptic, such as chlorhexidine or povidone-iodine solution. Neoplastic conditions of the anal sac may not allow anal sac expression and the instillation of antiseptic solutions. The patient is placed in a padded perineal stand (Figure 20-39). Proper position on the stand will help prevent circulatory compromise, neuropraxia and exacerbation of chronic osteoarthritis of the rear limbs. Several gauze sponges are placed into the patient’s rectum. The perineum is clipped and scrubbed according to acceptable standard aseptic techniques. The surgical site is draped routinely to protect the surgical wound.
In preparation for a closed anal sacculectomy some surgeons may elect to fill the anal sac with a groove director, self-hardening gel or resin, string, umbilical tape, plaster of Paris or dental acrylic to assist in the dissection of the anal sac and associated duct (Figure 20-40A). In larger dogs, a #6 Foley urinary catheter can be inserted into the anal sac and the balloon inflated. It should be noted that filling of the anal sac with material can only be performed in non-neoplastic diseases.
Based upon the described anatomy, a vertical skin incision is made over the anal sac, approximately 3 to 4 cm in length and 5 to 10 mm lateral to the mucocutaneous junction (Figure 20-40B and Figure 20-41). The subcutaneous tissue is incised equidistant with the skin. Blunt dissection and digital palpation is used to locate the anal sac. Blunt and sharp dissection using Steven’s tenotomy scissors, metzenbaum scissors and cottontipped applicators can be used to isolate the anal sac and duct (Figure 20-40C and Figure 20-42). Dissection of the anal sac from the internal and external anal sphincter can be difficult. The caudal rectal branch of the pudendal nerve should be avoided. Hemorrhage can be controlled using judicious use of electrocautery, ligatures and direct digital pressure. Once the anal sac and duct is dissected, the anal duct is ligated close to its termination at the anus and transected (Figure 20-40D). Alternatively, the anal duct can be transected at its termination at the anus (Figure 20-43). The anal mucosa is everted and subsequently closed using absorbable suture material (Figure 20-44).
Once transected, all tissue should be submitted for histopathological analysis. The surgical site should be lavaged thoroughly with sterile physiologic saline. The external anal sphincter should be closed using a synthetic, absorbable, monofilament suture, such as PDS or Maxon. The subcutaneous and subcuticular tissues should be apposed in a routine manner. If skin sutures are utilized, trim the ends of the sutures so that irritation to the adjacent anus and perineum will be minimized (Figure 20-40E and Figure 20-45). After surgery, a rectal exam should be performed. At this time, any defect in the rectal mucosa should be made apparent and if present, repaired appropriately.

Figure 20-39. A routine perineal stand involves placing the animal in ventral recumbency with the table slightly tilted forward. Adequate padding should be placed beneath the abdomen and at the caudal edge of the surgery table. The animal’s legs and tail are loosely tied to the surgery table.

Figure 20-40. Closed technique tor anal sac removal, A. A groove director is used to identify the anal sac. The anal sac can be filled to delineate it from surrounding tissues. B. An incision is made over the anal sac. C. The anal sac is dissected out from surrounding tissues, D. The duct is ligated, and the sac is removed, E. Routine closure. (Courtesy of Dr. Pamela Whiting.)
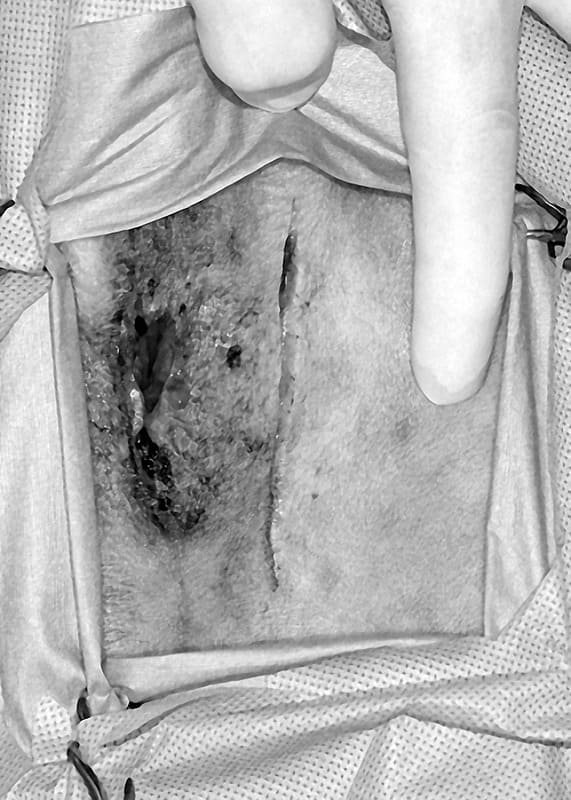
Figure 20-41. Initial skin incision, just lateral to the anus.

Figure 20-42. Dissection of the anal duct. Forceps are pointing to the anal duct. Note the presence of retractors in the surgical field.

Figure 20-43. Forceps are depicting the opening of the anal duct at the level of the inner cutaneous zone.

Figure 20-44. Apposition of the anal mucosa after transection of the anal duct.
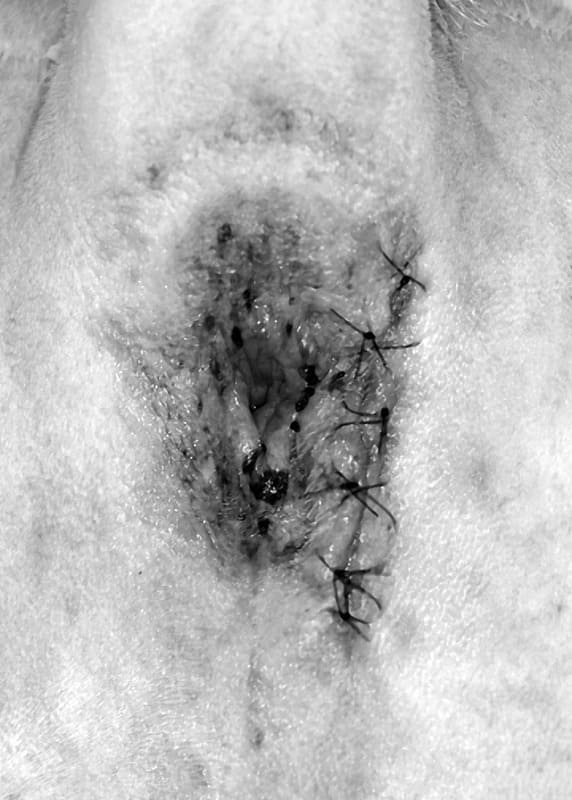
Figure 20-45. Apposed skin and anal mucosa. Note the short sutures along both apposed incisions.
Postoperative Care
An Elizabethan collar is recommended to prevent self-mutilation of the surgical site. Broad spectrum antibiotics should be administered immediately preoperatively and for the next 7 to 10 days due to the classification and location of the surgical wound. Cold compresses should be applied three to four times daily to the wound for the first 36 to 48 hours. After discontinuation of cold compresses, warm compresses should be applied two to three times daily until suture removal. Alternatively, hydrotherapy can be administered during the period of warm compresses. Hydrotherapy will not only help with any post-operative swelling, but will help keep the surgical site clean. Skin sutures, if present, are removed in 10 to 14 days.
Analgesics are paramount in the post-operative period. A full agonist opioid, such as hydromorphone or oxymorphone, should be utilized for the first 24 to 36 hours. If medically appropriate, a non-steroidal anti-inflammatory drug, such as carprofen or etodolac, can be administered for additional analgesia. A highfiber diet or stool softeners may be utilized to provide a soft, but formed stool. Either therapy can be useful in the post-operative period to help limit constipation associated with the administration of opioids or to help offset any potential tenesmus.
Preoperative conditions, such as hypercalcemia, should be monitored closely. If the hypercalcemia is secondary to a malignant neoplasm, such as an apocrine gland anal sac adenocarcinoma, the hypercalcemia should resolve if there is no local or distant tumor burden. However, if the hypercalcemia persists in the post-operative period, then either the tumor has metastasized or there is another disease process present. If the persistent hypercalcemia is secondary to a malignant neoplasm, consultation with a medical oncologist is recommended. Regardless of the presence of hypercalcemia, any patient suffering from a neoplasm of the anal sac or duct should be evaluated by a medical oncologist to determine if ancillary therapy is warranted.
Postoperative Complications
Short-term complications (< 14 days) after a closed anal sacculectomy include drainage, seroma formation, inflammation, hemorrhage, infection, and tenesmus or dyschezia. Drainage and seroma can be minimized by meticulous and delicate tissue handling and apposition of incised tissues. Inflammation can be minimized by avoidance of traumatic tissue handling, desiccation of exposed tissues, judicious use of electrocautery and proper identification of anatomy. Hemorrhage can be avoided if subcutaneous, muscular and parenchymal vessels are ligated using appropriate techniques and the use of electrocautery. If mild postoperative hemorrhage is present, a cold compress and sedation with acepromazine may provide relief. If hemorrhage is severe, immediate exploration of the surgical wound is indicated. Infection rates associated with a closed anal sacculectomy are low. However, if infection occurs, it will become apparent within the first 48 to 72 hours after surgery. The treatment of infection consists of removing the ventral sutures in the surgical wound to allow drainage and the application of dilute antiseptic solutions (chlorhexidine or povidone-iodine). Hydrotherapy performed twice daily and the administration of broad spectrum antibiotics (based on proper pharmacokinetics and suspected pathogen) pending results of bacterial culture and sensitivity testing is recommended.
Long-term complications of a closed anal sacculectomy can include fecal incontinence, chronic fistula formation, anal stricture and reoccurrence of local disease. The first three complications can be minimized with careful intraoperative technique and attention to anatomical structures. Fecal incontinence may result from excessive surgical trauma to the external anal sphincter (> 50% of its diameter) or direct damage to the caudal rectal branch of the pudendal nerve. Treatment of fecal incontinence includes dietary changes and the potential for muscle pedicle transpositions. Chronic fistula formation usually appears a few weeks to months after surgery. The cause of fistula formation is incomplete removal of the anal sac or duct. Treatment of the fistula includes surgical exploration of the draining tract and subsequent removal of any remaining secretory tissue. Anal stricture is an infrequent complication of closed anal sacculectomy due to the surgical approach. Clinically, anal strictures will appear weeks to months after surgery and clinical signs usually reveals tenesmus. Treatment of anal strictures includes stool softeners, balloon dilation, and if severe, surgical resection of the stricture. Local disease reoccurrence may occur in the case of anal sac neoplasms. Ancillary therapy, such as radiation therapy for local disease or chemotherapy for distant metastasis may be necessary for neoplasms. Complete staging of the neoplasm coupled with consultation with a medical oncologist is recommended.
Selected Readings
Aronson L. Rectum and anus. In: Slatter DH, ed. Textbook of small animal surgery. 3rd ed. Philadelphia: WB Saunders, 2002: 682-708.
Lipowitz A. Perineal Surgery. In: Lipowitz AJ, Caywood DD, Newton CD, et al, eds. Complications in small animal surgery. Baltimore: Williams & Wilkens, 1996: 527-540.
Van Sluijis FJ. Anal sacculectomy. In: van Sluijis FJ, ed. Atlas of small animal surgery. New York: Churchill Livingstone, 1992: 114-115.
Hill LN, Smeak D. Open versus closed bilateral anal sacculectomy for treatment of non-neoplastic anal sac disease in dogs: 95 cases (1969-1994). JAVMA 2002; 221: 662-665.
Van Duijkeren E. Disease conditions of canine anal sacs. JSAP 1995; 36: 12-16.
Nonsurgical Management of Perianal Fistulas
Dean Fillipowicz
Introduction
Dogs afflicted with anal furunculosis (perianal fistula, fistulae or fistulas; perianal hidradenitis) suffer from painful, malodorous and suppurative ulceration and sinus tract formation of the skin and subcutaneous tissues of the perineum. The etiology of this chronic and progressive inflammatory condition is unknown, but an immune mediated cause is likely. Previously regarded as a surgical disease, medical management is now the primary mode of treatment, with surgery reserved for recalcitrant cases and animals whose lesions are no longer responsive to immunosuppressive therapy. Goals of therapy include eliminating discomfort, ameliorating other associated clinical signs, and preventing recurrence.
Signalment and Clinical Signs
German Shepherd dogs are most commonly affected making up 89%,1 85%,2 81%,3 79%,4 96%,5 75%,6 and 100%7 of recent studies. Other breeds that have been reported include Labrador Retrievers, Irish Setters, Old English Sheep Dogs, Border collies, Bulldogs, Bouvier des Flandres, beagles, various spaniels, and mixed breeds.5,8-13 The mean age of presentation is between four and seven years old, but reports exist of patients aged between one and fourteen years old.12,13 A sex predisposition has not been substantiated; reports exist of increased male prevalence,4,7,14-18 increased female prevalence,19 and an equal sex distribution.3,6,20,21
Clinical signs most commonly reported include tenesmus, pain, dyschezia, excessive licking of the perineum, and a malodorous, purulent discharge, but self-mutilation, hematochezia, fecal incontinence, constipation or diarrhea, flatulence, and weight loss may also be seen.1-8,10,15,21
Patients present with varying degrees of ulceration and sinus tract formation radiating around the anus. In mild cases, the affected region may encompass an arc of 90° or less with focal, erythematous, superficial lesions. More advanced cases may have diffuse, deep, epithelial lined, communicating tracts extending 360° circumferentially with possible involvement of the anal sacs. True anocutaneous and rectocutaneous fistulae have been reported. Fortunately, they are uncommon in the canine species, with most dogs being presented with moderate to severe ulceration and multiple sinuses.5,10-13 Many dogs have advanced forms of the disease before initial presentation, possibly because the wide tail base and dense, thick hair coverage of many afflicted dogs prevents frequent observation of affected areas by owners.
Pathogenesis
Though the etiology of anal furunculosis is uncertain, several factors have been proposed as contributing to development of the disease: low tail carriage, broad tail base, and dense tail fur resulting in increased perianal humidity and contamination, anal sacculitis with concomitant spread of infection, anal crypt or gland fecalith impaction with subsequent abscessation, perianal trauma, and foreign body reaction. Though it is likely that these conditions may exacerbate inflammation and lesion formation, no strong evidence exists supporting any of these as a primary cause of the disease.2,9,10,13 Involvement of the anal sacs and subsequent infection, abscessation, and ulceration is common in advanced cases, but appears to be a secondary development rather than a primary cause of the disease.9,12 A correlation with hypothyroidism has been proposed, but remains unsubstantiated.17 In the same study, no immunologic abnormalities were found between affected and normal dogs.17
A failure in immune modulation is accepted to be the most likely cause of perianal fistulae, and evidence exists supporting this hypothesis. Most notably, anal furunculosis and Crohn’s disease in humans have similar clinical appearances and demonstrate similar positive responses to immunomodulatory medications.2-7,10,12,15,19,20,22 In addition, it has been shown that mRNA expression of those cyctokines associated with TH-1 T-cells is heightened in perianal tissue taken from dogs with anal furunculosis.23 However, a simple immunological defect, at least in German Shepherds, has not been found.24
Diagnosis and Evaluation
The diagnosis of anal furunculosis is based on signalment, history, physical examination findings, and exclusion of differential diagnoses. Severe local pain makes a thorough examination of the perineum difficult in affected animals and can seldom be done without general anesthesia. Prior to anesthesia, it is important to assess anal tone, as incontinence can be seen with advanced disease and as a post-operative complication. Once the patient is anesthetized, the perineal area should be liberally clipped to aid in assessment and subsequent cleaning of diseased tissue. Tracts should be gently probed with a sterile, blunt instrument to assess size, depth, and possible communication with nearby structures. At least one of the anal sacs is often secondarily involved or may become so before a response to therapy is seen. Both structures should be evaluated for involvement, rupture, or abscessation. Palpation, expression of the sacs if un-involved, and flushing with sterile saline to identify previously unobserved tracts is important. If occluded, the anal sac ducts should first be cannulated with a lacrimal duct cannula or small urinary catheter. Fine needle aspiration of grossly enlarged anal sacs may help identify abscessation or neoplasia.
A thorough rectal examination is necessary. The concurrent presence of a perineal hernia, or rectal dilation, or sacculation affects prognosis for both disease processes. Gently probing the fistulous tracts while performing the rectal examination may identify rectocutaneous fistulae. Care should be taken to identify thickening of the external anal sphincter and rectal and anocutaneous tissues, particularly in those patients suffering from tenesmus. Anorectal stenosis or stricture caused by chronic disease will also adversely affect prognosis.
Superficial cytology and culture gives little useful information, though culture and sensitivity of deep sinus tracts will aid in antibiotic selection for those cases responsive to medical management. Sinus tract biopsies can give histological support of a diagnosis of anal furunculosis, but more importantly, may identify neoplastic disease.
An association has been suggested between colitis and perianal fistulae.16,20 It is therefore further recommended to obtain colonic biopsies as the two disease entities present similarly, and treatment of fistulae is complicated by concurrent large bowel disease.
Additional diagnostics may include complete blood count, chemistry panel, and urinalysis for overall health assessment, fecal floatation to help identify endoparasites, and thoracic radiographs if neoplasia or fungal infection is suspected as contributing to the perianal ulceration. Other perineal disease entities to rule out include anal sac abscessation, fungal infection, pythiosis, lagenidiosis, perianal adenoma, apocrine gland adenocarcinoma, squamous cell carcinoma, caustic injury, and trauma.10,25
Medical Management-Systemic
It is important for owners to be aware that therapy is directed at control of the disease and its clinical signs and that a cure is seldom attained. In addition, owners must be well informed about the potential complications of management and should be committed to long-term aftercare.
Previously an exclusively surgically-managed disease, medical management is now the cornerstone of therapy for anal furunculosis. The goals of treatment are initially to eliminate discomfort and pain followed by reduction in lesion volume. Long term therapy is directed at prevention of disease recurrence. Four components make up medical management: regional hygiene, elimination/reduction of secondary infection, immunomodulatory therapy, and dietary modification. Surgery is indicated in recalcitrant cases and in those cases where no further improvement from medical management is noted. However, medical therapy should be be attempted first to reduce lesion severity; subsequent surgery may then be associated with fewer complications such as incontinence, stricture, and disease recurrence. 13,19
As therapy is initiated, attention to perianal hygiene should only be attempted with the aid of chemical restraint. As lesions and pain diminish, the patient may learn to tolerate disease care without sedation. Frequent clipping is initially necessary to remove debris and allow cleaning and monitoring of the affected area. Later in the course of management, the area should remain clipped and clean to prevent recurrence and allow application of topical medication.
Systemic antibiotics are indicated as most cases have some degree of secondary infection. Bacterial culture and sensitivity results should dictate the antibiotic of choice. However while results are pending, empiric therapy with an antibiotic that affords gram negative and anaerobic coverage such as amoxicillin-clavulanic acid is appropriate. Systemic therapy should continue for five days past the disappearance of gross evidence of infection. Topical antibiotic therapy such as mupirocin ointment (Bactoderm, Pfizer) once every twelve to twenty-four hours can be used once patient compliance allows application. This can be used to prevent and reduce bacterial colonization, and can be used concurrently with systemic antibiotics. Lesion resolution negates the need for continued antibiotic therapy. Hygiene and antibiotic therapy alone are unsuccessful in the management of this disease, and are considered to be palliative at best.9
Immunomodulatory therapy is the primary and most important aspect of the medical management of anal furunculosis, and several regimens are available. As discussed later, systemic cyclosporine coupled with ketoconazole followed by topical tacrolimus is the currently preferred method of management. However, reasonable success has been attained with cheaper, more commonplace immunosuppressives such as glucocorticoids, azathioprine, and metronidazole. Table (20-1) summarizes drug regimens recently as offering some success with less expensive drug combinations.
The advantages of glucocorticoid administration are ease and low cost. Unfortunately, side effects with this medication can be severe and include polydypsia, polyuria, polyphagia, decreased resistance to infection, slower wound healing, muscle wasting, and insulin antagonism. In addition, glucocorticoid administration for perianal fistulae may show no, poor, or transient response. Advantages of the metronidazole/azathioprine regimen include low cost, absence of untoward side effects, and reduced risk of post-operative complications previously reported.19 Though side effects were not seen in the cases of this report, this drug regimen is not innocuous. Azathioprine suppresses both humoral and cell mediated immunity and can result in gastrointestinal upset, pancreatitis, hepatotoxicity, and bone marrow suppression.26 Metronidazole is an antiprotozoal with immunomodulatory effects but can result in vomiting, anorexia, hepatotoxicity, and central vestibular signs.27

The advantages of glucocorticoid administration are ease and low cost. Unfortunately, side effects with this medication can be severe and include polydypsia, polyuria, polyphagia, decreased resistance to infection, slower wound healing, muscle wasting, and insulin antagonism. In addition, glucocorticoid administration for perianal fistulae may show no, poor, or transient response. Advantages of the metronidazole/azathioprine regimen include low cost, absence of untoward side effects, and reduced risk of post-operative complications previously reported.19 Though side effects were not seen in the cases of this report, this drug regimen is not innocuous. Azathioprine suppresses both humoral and cell mediated immunity and can result in gastrointestinal upset, pancreatitis, hepatotoxicity, and bone marrow suppression.26 Metronidazole is an antiprotozoal with immunomodulatory effects but can result in vomiting, anorexia, hepatotoxicity, and central vestibular signs.27
The similarities in clinical appearance between Crohn’s disease in humans and canine anal furunculosis led to the discovery that encouraging treatment results can be achieved when affected dogs are treated with the same immunomodulating drug used in human medicine.2-7,10,14,15,22,28 Cyclosporine acts by reversible inhibition of calcineurin, an enzyme normally partially responsible for cytokine synthesis, among other functions. The end result is inhibition of cell mediated immunity and T-cell (T-helper and T-cytotoxic) activation and proliferation.10,22 The microemulsion (ME) formulation of this drug (Atopica, Novartis) is given because of improved bioavailability and decreased inter-individual serum levels. Because of delayed absorption when given with food (even with the ME formulation), it is recommended that cyclosporine be administered two hours before or after a meal.22
Several reports have shown the benefits of the sole use of cyclosporine in the treatment of anal furunculosis, and several conclusions have been reached: faster remission and higher recovery rates are seen with higher dosages, clinical signs may be more likely to return after cessation of high dose treatment, and longer administration (thirteen weeks) decreases the rate of relapse.2,4-7,11,14,15,29 Unfortunately, a definitive dosing regimen has not been found. A recent review recommends initially treating at 4 to 8 mg/kg PO q 24h for eight to sixteen weeks until a marked resolution of clinical signs has occurred. At that point, it has been suggested to decrease the dose by 20 to-40% or decrease the frequency of administration to every forty-eight hours with further tapering based on clinical response and lack of recurrence.10
Though encouraging results have been obtained with the use of cyclosporine, difficulties still exist as to the appropriate dosage, schedule, and duration of therapy. Initial dose schedules were extrapolated from human medicine where cyclosporine was used to prevent renal allograft rejection. Because of the high doses required to prevent organ rejection and the relatively small margin of safety this drug has in humans (hypertension and nephrotoxicity are not uncommon side effects), dosing adjustments in transplant patients are necessary and are made based on serum trough levels. The treatment of canine anal furunculosis requires lower doses of cyclosporine than is required to prevent organ rejection. In addition, recent studies in dogs with anal furunculosis treated with cyclosporine failed to find a relationship between trough concentrations and treatment efficacy.5,7 Therefore, though monitoring trough levels in transplant patients assists in fine tuning those treatment regimens, the practice provides no additional information in the treatment of most cases of anal furunculosis in which a favorable clinical response is seen to cyclosporine alone.
Serious side effects are rare with cyclosporine administration in dogs, but long term studies are needed to assess its full effects.22 Side effects noted in recent clinical reports include hair shedding that may be followed by increased hair growth, gastrointestinal effects (vomiting and diarrhea, inappetance), lethary, and lameness.2-5,15 The most common side effects, hypertrichosis and vomitting/diarrhea, were mild and resolved spontaneously during treatment or after cyclosporine administration had stopped. Gingival hyperplasia and papillomatosis have also been reported.28 Though there is an increased risk in humans of developing lymphoma after cyclosporine usage, there are only two case reports in the veterinary literature (one cat after renal transplant surgery and one dog with anal furunculosis) that suggest lymphomagenesis may be associated with cyclosporine administration.30 There is no evidence that administration of cyclosporine in dogs, or in humans with dermatologic conditions, has been associated with an increased risk of infection.22
Unfortunately, cyclosporine is expensive, particularly when treating the large breeds affected by anal furunculosis. By inhibiting the cytochrome P450 system, ketoconazole has been used to decrease the hepatic clearance of cyclosporine resulting in increased serum levels. Lesser amounts of cyclosporine are then needed at a significant cost advantage to the owner. Increases in serum cyclosporine levels are proportional to ketoconazole doses when the latter is dosed between two and twelve mg/kg.31 Unfortunately, the amount of increase in cyclosporine blood concentration due to hepatic inhibition from ketoconazole is individually variable, necessitating dose adjustments for most patients. This is an indication for measuring cyclosporine serum trough levels. Trough levels can be evaluated by high pressure liquid chromatography (HPLC), fluorescent polarization immunoassay (FPIA) or radioimmunoassay (RIA). These last two assays, though faster and cheaper, use antibodies that cross react with cyclosporine metabolites, and can overestimate the cyclosporine blood concentration. In fact, cyclosporine blood concentrations when measured with FPIA are nearly twice those measured with HPLC. This latter assay is more expensive and less widely available, but more accurate.22
A previous review suggests an initial starting dose of ketoconazole at 5-10 mg/kg PO q 24h in conjunction with a moderate starting dose of cyclosporine at 5mg/kg PO q 24h.10 With resolution of lesions and other clinical signs, the cyclosporine dose is tapered starting six to ten weeks after initiation of therapy. Higher doses may be required in some recalcitrant cases or in more severe, chronic cases. The goal of therapy is the lowest dose and frequency of both drugs that will prevent recurrence of lesions or clinical signs. Some animals may only require topical medication (described below) as maintenance therapy, while others will require life long cyclosporine treatment with or without topical therapy. Trough cyclosporine levels, measured ideally with HPLC, should be assessed if the patient is not or is no longer responding to treatment. Dosing should be increased in these cases if trough levels below 400 ng/ml are found. Serum trough levels to monitor dose reductions are also necessary when signs of cyclosporine toxicosis are noted.
Because large variations (10 to 60%)4,7 in blood cyclosporine concentrations exist between dogs on the same dose of cyclosporine given both cyclosporine and ketoconazole for the treatment of anal furunculosis, definitive dosing regimens are unavailable, and the above is given as a starting point. However, encouraging results have been reported with the use of this drug combination (Table 20-2).
Side effects noted in clinical reports of combination therapy included vomiting, diarrhea, inappetance, weight loss, hypertrichiasis, hypoalbuminemia, lameness, and gingival hyperplasia. Most effects were transient during initial treatment and resolved spontaneously or with minimal intervention. Those side effects which were more recalcitrant resolved with cessation or reduction of administration and were not intolerable.4,6,7,32 Hepatotoxicity is a suggested side effect of ketoconazole and may be idiosyncratic or dose dependent, though none of the previous reports describe this complication.33
Medical Management-Topical
In 2000, Misseghers et al.reported on once to twice daily topical application of tacrolimus (0.1%) to treat canine anal furunculosis in ten dogs.34 Like cyclosporine, this drug is used to inhibit T-cell activation through inhibition of calcineurin. Tacrolimus is ten to one hundred times more potent than cyclosporine, is absorbed topically better than cyclosporine, and doesn’t require systemic levels to be effective. Side effects are typically reported at the same frequency as those of cyclosporine. However none of the animals described in the initial study showed untoward side effects. Five of the ten dogs (50%) achieved full remission of between one and eight months duration after cessation of treatment, and nine of ten (90%) dogs showed complete resolution of clinical signs with at least a 50% reduction in lesion volume. This report suggested that once daily administration of tacrolimus should be considered a low cost alternative to cyclosporine in mild to moderate cases of anal furunculosis or as maintenance therapy after cyclosporine induced remission of disease.
There is little in the literature that describes the concurrent use of both calcineurin inhibitors, but combination therapy should have a role in treatment. One group has reported good results in “normal” cases of anal furunculosis with twice daily application of tacrolimus that was started once tapering of cyclosporine has begun.10 Anecdotally, the tacrolimus seems to speed cyclosporine tapering. This paper suggests that some patients can be weaned entirely off systemic cyclosporine/ketoconazole and be managed with tacrolimus applied topically every 24 to-72 hours. In very mild cases of disease, tacrolimus alone may be sufficient. Whether topical medication may be required for life long management10 or should only be continued for four weeks past resolution of clinical signs34 in these cases is uncertain. If tacrolimus is not used because of client or patient concerns, the lowest dose and frequency of cyclosporine and ketoconazole (or other immunomodulator combination) that controls clinical signs is recommended.
Dietary Management
To my knowledge, there have been no prospective studies which show a definitive response to restrictive diets. However, anecdotally, many authors agree that the introduction of a novel antigen diet is a component of treatment.10,16,20,25 An association between colitis and anal furunculosis has been suggested.16 Thirty-three percent (9/27) of dogs with perianal fistulas in a study of 27 German Shepherd dogs with a concurrent diagnosis of colitis experienced complete lesion resolution, 33% experienced an improvement in lesion severity, and the remainder were unchanged16 when treated with corticosteroids and a novel protein diet. In another study, 50% (9/18) of dogs had histological diagnoses of colitis when they were presented for perianal fistulae. Several theories have been proposed for this association, but our lack of understanding of either disease precludes a definitive etiology without further study.
Summary
Life long medical management can reduce the severity of or prevent the recurrence of clinical signs. Systemic and/or topical medication have shown good results in decreasing the severity of disease. The importance of recheck evaluations should be stressed to owners. Rectal examinations should be performed biannually unless there are clinical problems that recur sooner. Adjunctive surgical intervention should only be attempted after medical options have been explored and owners have been advised of possible surgical complications.
Anal furunculosis has historically been a surgically managed disease. Surgery is still indicated in those rare cases that are completely unresponsive to immunomodulators and in those in which no further response is appreciated despite appropriate drug dosing adjustments. The trend away from surgical therapy has been a result of the high rates of fistulae recurrence and potential serious surgical complications regardless of the technique employed.
References
- Milner, H.R., The role of surgery in the management of canine anal furunculosis. A review of the literature and a retrospective evaluation of treatment by surgical resection in 51 dogs. N Z Vet J, 2006. 54(1): p. 1-9.
- House, A.K., et al., Evaluation of the Effect of Two Dose Rates of Cyclosporine on the Severity of Perianal Fistulae Lesions and Associated Clinical Signs in Dogs. Veterinary Surgery, 2006. 35(6): p. 543-549.
- Hardie, R.J., et al., Cyclosporine treatment of anal furunculosis in 26 dogs. J Small Anim Pract, 2005. 46(1): p. 3-9.
- O’Neill, T., G.A. Edwards, and S. Holloway, Efficacy of combined cyclosporine A and ketoconazole treatment of anal furunculosis. J Small Anim Pract, 2004. 45(5): p. 238-43.
- Doust, R., L.G. Griffiths, and M. Sullivan, Evaluation of once daily treatment with cyclosporine for anal furunculosis in dogs. Veterinary Record, 2003. 152(8): p. 225-+.
- Patricelli, A.J., R.J. Hardie, and J.E. McAnulty, Cyclosporine and ketoconazole for the treatment of perianal fistulas in dogs. J Am Vet Med Assoc, 2002. 220(7): p. 1009-16.
- Mouatt, J.G., Cyclosporin and ketoconazole interaction for treatment of perianal fistulas in the dog. Aust Vet J, 2002. 80(4): p. 207-11.
- Ellison, G.W., Treatment of perianal fistulas in dogs. J Am Vet Med Assoc, 1995. 206(11): p. 1680-2.
- Killingsworth, C.R., et al., Bacterial population and histologic changes in dogs with perianal fistula. Am J Vet Res, 1988. 49(10): p. 1736-41.
- Patterson, A.P. and K.L. Campbell, Managing anal furunculosis in dogs. Compendium on Continuing Education for the Practicing Veterinarian, 2005. 27(5): p. 339-+.
- Mathews, K.A., et al., Cyclosporine Treatment of Perianal Fistulas in Dogs. Can Vet J, 1997. 38: p. 39-41.
- Day, M.J. and W.B. M., Pathology of Surgically Resected Tissue from 305 Cases of Anal Furunculosis in the dog. J Small Anim Pract, 1992. 33: p. 583-589.
- Matushek, K.J. and E. Rosin, Perianal Fistulas in dogs. Compendium on Continuing Education for the Practicing Veterinarian, 1991. 13(4): p. 621-627.
- Griffiths, L.G., M. Sullivan, and W.W. Borland, Cyclosporin as the sole treatment for anal furunculosis: preliminary results. J Small Anim Pract, 1999. 40(12): p. 569-72.
- Mathews, K.A. and H.R. Sukhiani, Randomized controlled trial of cyclosporine for treatment of perianal fistulas in dogs. J Am Vet Med Assoc, 1997. 211(10): p. 1249-53.
- Harkin, K.R., R. Walshaw, and T.P. Mullaney, Association of perianal fistula and colitis in the German shepherd dog: response to high-dose prednisone and dietary therapy. J Am Anim Hosp Assoc, 1996. 32(6): p. 515-20.
- Killingsworth, C.R., et al., Thyroid and immunologic status of dogs with perianal fistula. Am J Vet Res, 1988. 49(10): p. 1742-6.
- Vasseur, P.B., Results of surgical excision of perianal fistulas in dogs. J Am Vet Med Assoc, 1984. 185(1): p. 60-2.
- Tisdall, P.L., et al., Management of perianal fistulae in five dogs using azathioprine and metronidazole prior to surgery. Aust Vet J, 1999. 77(6): p. 374-8.
- Jamieson, P.M., et al., Association between anal furunculosis and colitis in the dog: preliminary observations. J Small Anim Pract, 2002. 43(3): p. 109-14.
- Ellison, G.W., et al., Treatment of perianal fistulas with ND:YAG laser- -results in twenty cases. Vet Surg, 1995. 24(2): p. 140-7.
- Guaguere, E., J. Steffan, and T. Olivry, Cyclosporin A: a new drug in the field of canine dermatology. Vet Dermatol, 2004. 15(2): p. 61-74.
- House, A.K., S.P. Gregory, and B. Catchpole, Expression of Cytokine mRBA in Canine Anal Furunculosis Lesions. Veterinary Record, 2003. 153(354-358).
- Day, M.J., Immunopathology of Anal Furunculosis in the Dog. J Small Anim Pract, 1993. 34: p. 381-389.
- Ettinger, S.J. and E.C. Feldman, Textbook of Veterinary Internal Medicine. 5th ed. Diseases of the Large Intestine, ed. A.E. Jergens and M.D. Willard. Vol. 2. 2000, Philadelphia: W. B. Saunders.
- Plumb, D.C., Veterinary Drug Handbook. 4 ed. Azathioprine, ed. D.C. Plumb. 2002, Ames: Iowa State University Press. 86-88.
- Plumb, D.C., Veterinary Drug Handbook. 4 ed. Metronidazole, ed. D.C. Plumb. 2002, Ames: Iowa State University Press. 549-552.
- Daigle, J.C., More economical use of cyclosporine through combination drug therapy. Journal of the American Animal Hospital Association, 2002. 38(3): p. 205-208.
- Hardie, R.J., et al., Cyclosporine Treatment of Perianal Fistulae in 26 Dogs. Vet Surg, 2000. 29(5): p. 481.
- Blackwood, L., et al., Multicentric lymphoma in a dog after cyclosporine therapy. J Small Anim Pract, 2004. 45(5): p. 259-62.
- Myre, S.A., T.J. Schoeder, and V.R. Grund, Critical Ketoconazole Dosage Range for Ciclosporin Clearance Inhibition in the Dog. Pharmacology, 1991. 43: p. 233-241.
- Dahlinger, J., C. Gregory, and J. Bea, Effect of ketoconazole on cyclosporine dose in healthy dogs. Veterinary Surgery, 1998. 27(1): p. 64-68.
- Plumb, D.C., Veterinary Drug Handbook. 4 ed, ed. D.C. Plumb. 2002, Ames: Iowa State University Press. 465-470.
- Misseghers, B.S., A.G. Binnington, and K.A. Mathews, Clinical observations of the treatment of canine perianal fistulas with topical tacrolimus in 10 dogs. Can Vet J, 2000. 41(8): p. 623-7.
Excisional Techniques for Perianal Fistulas
Gary W. Ellison
Introduction
Since the last edition of this text was published, dramatic improvements have been made in the medical management of perianal fistulas to the point that surgical therapy is currently considered a secondary treatment for this confounding disease. Current theory is that the disease may be immune mediated resembling Crohns disease in people. The term “immune mediated proctitis” may be a better term for this syndrome than perianal fistulas. The presence of concomitant inflammatory bowel disease and recent reported success of immunosuppressive therapy with drugs such as oral prednisone, oral cyclosporine, combination oral cyclosporine and ketoconizole, combination oral azathioprine with metronidazole and topical tacrolimus lend further support to this hypothesis. Early infiltration of the circumanal glands with lymphocytes and plasma cells may occur prior to the more traditional secondary histologic changes which include inflammation and necrosis of the apocrine glands, infection of circumanal glands or hair follicles, impaction and infection of the anal sinuses or anal crypts and anal sac infection or abscessation. Ultimately the normal regional anatomy is permanently disrupted and gross lesions include fistulas of the anal sinuses, submucosal fistulas and ruptured anal sacs in addition to cutaneous perianal fistulas. In these advanced cases medical therapy is more likely to fail and surgery is indicated.
Indications for Surgery
Although an estimated 85% to 90% of perianal fistulas show improvement or complete resolution after eight to twelve weeks of immunosuppressive therapy, those lesions associated with anal sac rupture or disruption of the anal sac ducts often will not completely respond, or are subject to recurrence after cessation of appropriate medical therapy. Recurrence rates of 40 to 60% are reported especially in those cases where anal sac drainage is impaired. In the authors experience, residual or recurrent lesions are most often located ventral and lateral to the anus. Surgical excision of fistulas with concurrent anal sacculectomy is indicated in these cases as well as those where large areas of the anal circumference are involved and continued tenesmus or dyschezia with obstipation is caused by anal stenosis. Potential limitations of excision include the inability to remove all of the tracts if they extend too far peripherally and the danger of creating fecal incontinence if the tracts deeply invade the external anal sphincter. However, if an anal stricture is present due to a deep-seated invasion of the external anal sphincter by the fistulas, or if extensive fibrosis has occurred, then incision or excision of the areas of fibrosis is usually necessary for release of the constriction and for relief of painful defecation.
Preoperative Medical Treatment
For recurrent fistulas I prefer to treat the patient for two to four weeks preoperatively with 2 to 3 mg/kg of cyclosporine (Neoral, Novartis NA, East Hanover, NJ) PO every 12 hours or with a combination of 0.25 to 0.5 mg/kg cyclosporine and 10 mg/kg of ketaconazole (Nizoral, Janssen, Titusville, NJ) PO once daily. Pre-treatment CBC and blood chemistries should be drawn to establish baseline values for liver and renal function. Cyclosporine dosages often need to be adjusted to achieve a target range concentration of 200 ng/L on a 12 hour trough serum sample. The goal of presurgical therapy is to reduce existing fistulas to a negligible size and therefore reduce the volume of diseased tissue that needs to be excised. Reduction in diseased tissue will also allow the surgeon to better visualize the involved anal sacs and aid in their complete removal. If perianal fistula disease is chronic in a dog, abdominal radiographs should be taken to rule out the presence of secondary megacolon. Biopsies may be indicated in some cases to rule out neoplastic disease before extensive surgical therapy is performed.
Surgical Technique
The perianal area and tail base are clipped extensively after the animal has been placed under general anesthesia. A thorough digital rectal examination should be performed to determine how much of the rectal circumference is diseased, to identify the severity of anal sphincter stenosis and to determine how far the fistulas extend peripherally. The fecal contents of the rectum are evacuated digitally. Enemas are usually not administered prior to surgery unless significant fecal impaction is present. Culture of the fistulous tracts usually is not warranted since a mixed culture of gram positive cocci and gram negative coliform bacteria are usually isolated. The animal can be positioned in ventral or dorsal recumbency with the tail pulled over the back or below the table, respectively. The rectum is packed with chlorhexidine soaked tampons and routine surgical preparation of the perianal region is performed.
The fistulas and anal sacs are carefully probed with a groove director to determine their extent and depth. When the anal sacs are not diseased, they are removed prior to fistulectomy. More commonly, the anal sacs are ruptured or abscessed, and are best excised concurrently with the fistulous tracts. A circular incision is made around the periphery of the fistulas using a #10 blade, needle tipped electrosurgical unit or surgical laser (Figure 20-46). A plane is established deep to the fistulas and dissection is carried medially toward the anal canal. The dissection plane must stay as close to the fistulas as possible to preserve the external anal sphincter but it is important to dissect deep to the fibrous tracts (Figure 20-47). Hemorrhage is moderate and is controlled with electrocoagulation or laser ablation. Any remaining anal sac lining is carefully dissected from the surrounding fibers of the external anal sphincter with mosquito hemostats or fine dissection scissors (See Figure 20-47 inset). The entire secretory lining of the anal sac must be removed or sinus tracts may develop postoperatively. The dissection is carried medially to the anal canal and a circular incision is made in healthy rectal mucosa cranial to any rectal or anal sinuses (Figure 20-48). The excised fistulous tracts and a portion of rectal mucosa should be submitted for histopathology to rule out neoplasia and to check for evidence of inflammatory bowel disease. Eight to ten simple interrupted sutures of 3-0 synthetic monofilament absorbable sutures are used to appose the rectal submucosa to the subcutis. The rectal mucosa is then sutured to the skin with simple interrupted 3-0 monofilament nylon or polypropylene sutures (Figure 20-49 and inset). Often there are areas where the fistulas extend so far peripherally that direct skin to mucosal apposition is not possible. In these cases the adjacent areas of skin can be apposed or the wound can be left open to heal by second intention.
Laser excision–A Nd:YAG contact-tip laser has been used to successfully treat perianal fistulas. A frosted, synthetic sapphire tip and a continuous impulse of 13 to 15 watts was used to excise the fistulas, and the wound was closed primarily. Anal tone was reduced, flatulence was increased in 60% of the dogs, and 20% developed fecal incontinence. However, fecal incontinence when present was effectively managed by means of diet modification. The overall success rate for resolution of fistulas was 95% during a mean follow-up time of 22.9 months. Surgical treatment by laser excision of fistulas was particularly effective in relieving pain in those dogs with preexisting anal stenosis. Other authors have successfully used the CO2 laser for fistulectomy.
Figure 20-46. A groove director is used to probe the fistulous tracts and also check for patency of the anal sacs. An incision is made around the perimeter of the tracts with a #10 scalpel, needle tipped electrosurgical unit or laser unit.

Figure 20-47. The dissection is continued deep to the tracts with efforts at preserving as much of the anal sphincter. Residual anal sacs lining should be removed by blunt dissection using mosquito hemostats or fine tipped scissors (inset).

Figure 20-48. Cross section of the anus and rectum showing excision of the fistulas with preservation of the external anal sphincter. The fistulas are transected through the rectal mucosa cranial to the anocutaneous junction.
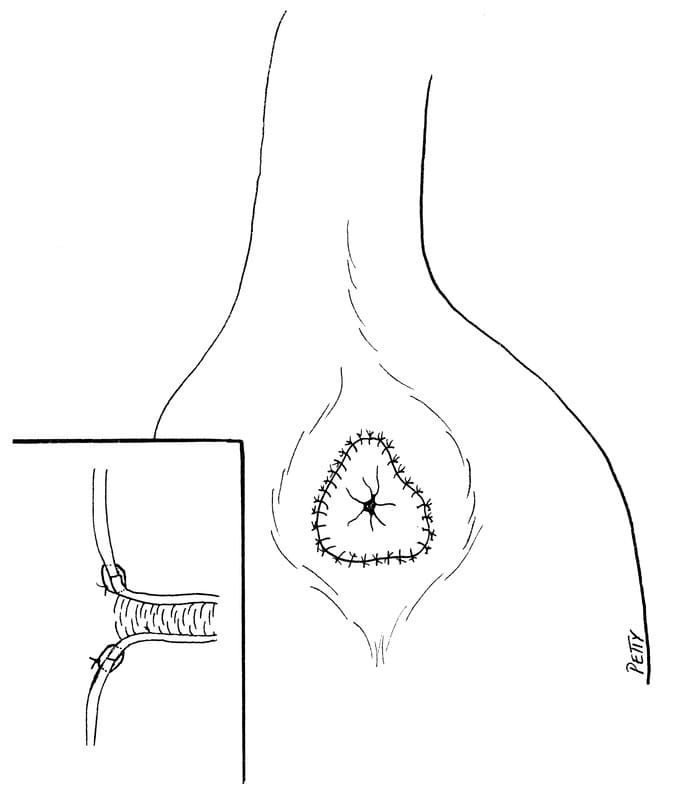
Figure 20-49. After apposing the deep subcutaneous tissues with simple interrupted sutures the rectal mucosa is apposed to the skin with simple interrupted sutures. It is important to closely approximate the rectal mucosa with the skin (inset).
Postoperative Care and Complications
Dogs may experience significant postoperative pain the day of surgery and injectable morphine, hydromorphone or bupivacaine or morphine epidural analgesia is often prescribed in addition to injectable NSAIDS (See Chapter 9). NSAIDS should not be administered to patients that have recently received prednisone. Pain usually resolves rapidly and often within 48 hours animals are less painful than they were preoperatively. Drainage of serosanguineous fluid from the wound edge is expected for several days. Perioperative antibiotics may be indicated since fecal contamination may occur during surgery. Intravenous cefazolin (22 mg/ kg IV every 8 hours) in combination with metronidazole (15 mg/ kg IV every 8 hours) is initiated prior to surgery and continued for 24 hours. Oral cephalexin (10 mg/kg every 12 hours) and metronidazole (15 mg/kg every 12 hours) is then continued for five days postoperatively. The area is gently cleaned twice daily and sutures are removed in 10 days. Partial wound dehiscence is not uncommon. If wound dehiscence occurs, it is managed by local wound flushes and parenteral antibiotics as needed. Most open wounds heal by second intention in two to three weeks. Stool softeners are only used if preoperative constipation was present. Various degrees of fecal incontinence or flatulence may occur after the procedure. Fecal incontinence is usually less common with first time surgical procedures, but tends to occur more commonly when multiple procedures have been performed. When present, fecal incontinence is often successfully managed by feeding diets with high digestibility. The reported long-term success rates of excision techniques varies from 46 to 95%. However, many animals undergoing surgery have had other procedures performed prior to the excision technique. Postoperative anal strictures are rare with this technique. Periodic clipping and daily cleaning of the perianal region should be performed by the owners during the remainder of the dog’s life. All dogs should undergo reexamination every two months to look for early signs of recurrence. Recurrent superficial ulcerations when treated early, usually respond to two to four weeks of cyclosporine therapy without further surgery.
Suggested Readings
Day MJ, Weaver BMQ: Pathology of surgically resected tissue from 305 cases of anal furunculosis in the dog. J Sm Anim Pract 33:583, 1992.
Ellison GW: Treatment of perianal fistulas in dogs. J Am Vet Med Assoc 206:1680, 1995.
Ellison GW, Bellah JR, Stubbs WP: Treatment of perianal fistulas with ND/YAG laser-results of 20 cases. Vet Surg 24:140, 1995.
Harkin KR, Walshaw RW, Mullaney TP: Association of perianal fistula and colitis in the German Shepherd dog: Response to high dose prednisone and dietary therapy. J Am Anim Hosp Assoc 35:515, 1996.
Mathews KA, Sukiana HR: Randomized controlled trial of cyclosporine for treatment of perianal fistulas in dogs. J Am Vet Med Assoc 211:1249, 1997.
Misseghers BS, Binnington AG, Matthews KA: Clinical observations in the treatment of perianal fistulas with topical tacrolimus in 10 dogs. Can Vet J 41:623, 2000.
Mouatt JG: Cyclosporin and ketaconazole interaction for treatment of perianal fistulas in the dog. Aust Vet J 80:207, 2002.
Shelley BA: Use of the carbon dioxide laser for perianal and rectal surgery. Vet Clin North Am Small Anim Pract 32:621, 2002.
Tisdale PL, Hunt GB, Beck JA, et al: Management of perianal fistulae in five dogs using azathioprine and metronidazole prior to surgery. Aust Vet J 77:374, 1999.
Vasseur PB: Results of surgical excision of perianal fistulas in dogs. J Am Vet Med Assoc 185:60, 1984.
Get access to all handy features included in the IVIS website
- Get unlimited access to books, proceedings and journals.
- Get access to a global catalogue of meetings, on-site and online courses, webinars and educational videos.
- Bookmark your favorite articles in My Library for future reading.
- Save future meetings and courses in My Calendar and My e-Learning.
- Ask authors questions and read what others have to say.
About
How to reference this publication (Harvard system)?
Author(s)
Copyright Statement
© All text and images in this publication are copyright protected and cannot be reproduced or copied in any way.Related Content
Readers also viewed these publications
Buy this book
Buy this book
This book and many other titles are available from Teton Newmedia, your premier source for Veterinary Medicine books. To better serve you, the Teton NewMedia titles are now also available through CRC Press. Teton NewMedia is committed to providing alternative, interactive content including print, CD-ROM, web-based applications and eBooks.
Teton NewMedia
PO Box 4833
Jackson, WY 83001
307.734.0441
Email: [email protected]









Comments (0)
Ask the author
0 comments