

Get access to all handy features included in the IVIS website
- Get unlimited access to books, proceedings and journals.
- Get access to a global catalogue of meetings, on-site and online courses, webinars and educational videos.
- Bookmark your favorite articles in My Library for future reading.
- Save future meetings and courses in My Calendar and My e-Learning.
- Ask authors questions and read what others have to say.
Electrosurgery and Laser Surgery
Get access to all handy features included in the IVIS website
- Get unlimited access to books, proceedings and journals.
- Get access to a global catalogue of meetings, on-site and online courses, webinars and educational videos.
- Bookmark your favorite articles in My Library for future reading.
- Save future meetings and courses in My Calendar and My e-Learning.
- Ask authors questions and read what others have to say.
Read
Electrosurgical Techniques
Robert B. Parker
Electrosurgical units are probably among the most frequently used and least understood surgical instruments. Little information is available in the veterinary literature concerning basic electronics, proper surgical techniques, and potential hazards. Judicious use of electrosurgery can be of great benefit to the veterinarian in maintaining a bloodless surgical field, but indiscriminate use can create serious complications. The following discussion describes available electrosurgical methods and apparatus and provides a guideline for their proper use.
Electrolysis
Electrolysis implies a unidirectional, direct current flow that produces strong polarity in the anode and cathode (Figure 3-1). The system is of low voltage and amperage. When the electrodes are inserted into the body, hydroxides are produced at the treatment cathode by the following formula:
2 NaCl + 4 H20 -> 2 NAOH + 2 H2 (cathode)
-> 2 HCI + O2 (anode)
The hydroxides liquefy tissue, yet produce minimal discomfort. Electroepilation has been used in ophthalmic surgery for treatment of ectopic cilia or distichiasis. The fine cathode electrode is passed to the base of the cilia, where the current and hydroxides liquefy and destroy the ciliary root.

Figure 3-1. Basic circuit diagram for an electrolysis unit.
Electrocautery
The use of cautery to control hemorrhage dates back to ancient times, when a hot iron was used to cauterize wounds. More sophisticated microcautery is now available, but the technique of direct heat application is the same.
Low voltage current is used to heat the treatment electrode, and therefore, electrical energy does not pass through the body (Figure 3-2). The destructive effect is heat coagulation, and the temperature is proportional to the intensity of the current flowing through the resistance of the tip.
Advantages of this technique are that 1) the degree of tissue damage is apparent, 2) it coagulates well in a bloody field, and 3) it is inexpensive and simple. The disadvantages are that 1) tissue destruction can be extensive and 2) large lesions are slowly destroyed.
Electrocautery units are generally reserved for minor surgical procedures, such as dewclaw or tail removal in puppies. Disposable electrocautery units, frequently used in ophthalmic surgery, provide fine hemostasis by pinpoint heat application (Figure 3-3).
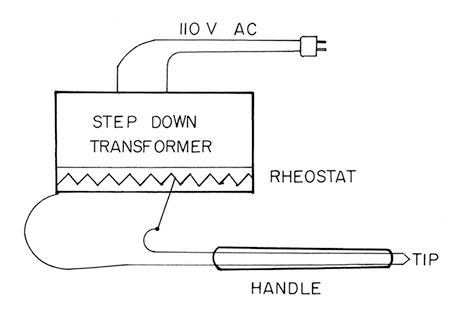
Figure 3-2. Basic circuit diagram for a thermal electrocautery unit.

Figure 3-3. Disposable electrocautery unit.
High Frequency Electrosurgery
Most electrosurgical units available today fall into this category. The unit is essentially a radio transmitter that produces an oscillating high frequency electrical field of 500,000 to 100,000,000 hertz (cycles per second). Above 10,000 hertz, current can be passed through the body without pain or muscle contraction. In contrast to electrocautery, the treatment electrode is not hot, but serves to deliver electrical energy at a concentrated area. The electrosurgical effect is determined by 1) the tissue resistance, 2) the mode of application, and 3) the amount and type of current. These factors can be modified to produce the desired surgical response.
Body tissue and fluids have a definite electrical impedance or resistance. Heat is produced by the resistance to current flow as electrical energy is absorbed and converted to thermal energy. Because resistance is inversely proportional to surface area, resistance decreases as the current spreads over the body.
The mode of application can be either uniterminal or biterminal. Biterminal application, used most frequently with cutting or coagulation, implies the use of an indifferent electrode or “ground plate” (Figure 3-4). The indifferent electrode collects the current when it has passed through the body and dissipates it over a large surface area to produce a low current density. Because heat production is inversely proportional to the contact area, the large size of the indifferent electrode evenly distributes the heat to prevent burning. The active electrode concentrates the same energy at a small point and produces the surgical effect (Figure 3-5).
With the uniterminal technique, the patient is not incorporated into the electrical circuit. An indifferent electrode is not used and the electrical energy is absorbed by the patient and is radiated into the air. Thus, sparking is produced at the tip and is directly applied to the lesion to cause either fulguration or desiccation (See Figure 3-4).

Figure 3-4. Uniterminal techniques, electrofulguration A. and electrodes- iccation B. Biterminal techniques, electrotomy and electrocoagulation C.

Figure 3-5. High current density at the active electrode and low current density with a properly placed indifferent electrode.
Most modern electrosurgical units provide different waveforms to bring about either cutting or coagulation. An undamped, continuous sine wave makes the most effective cutting current (Figure 3-6). Little hemostasis is achieved with a pure sine wave. In older units, a triode vacuum tube was used to produce the sine wave, but newer solid state units use electronic circuitry to yield a more refined current. A series of damped or interrupted waves achieve coagulation with limited cutting capability (Figure 3-7). Blended currents are possible and produce a combined cutting and coagulation mode (Figure 3-8). The more expensive units are capable of varying the “on-to-off” time to accomplish degrees of cutting versus coagulation.

Figure 3-6. Undamped, continuous sine (cutting) waves.

Figure 3-7. Damped (coagulation) waves.

Figure 3-8. Blended (combined cutting and coagulation) waves.
Surgical Techniques
These techniques include electrotomy, electrocoagulation, and electrofulguration and electrodesiccation.
Electrotomy
Electroincision of any tissue causes greater tissue damage than sharp incision; therefore, the veterinarian must weigh the advantages of reduced blood loss and operating time against the disadvantages of increased tissue destruction and healing time. Electroincision of the skin heals primarily, but a definite lag is seen in the ultimate healing of the wound. Healing does occur, however, and maximal breaking strength is achieved.
The primary indications for electroincision of the skin are in patients with clotting disorders or when anticoagulant treatment is anticipated, such as with cardiopulmonary bypass procedures. Because of the initial delay in wound healing, it is recommended that skin sutures remain approximately 2 to 3 days longer with a skin incision made with an electrosurgical unit. The amount of coagulation and necrosis is proportional to the amount of heat produced and its duration of contact. Therefore, it is best to use a smooth, swift stroke when using an electrosurgical scalpel.
The high frequency electrosurgery units such as the Ellman Surgitron (Ellman International, Hewlett, NY) cause no more tissue destruction than traditional cold scalpel surgery if used in the pure cutting mode.
An electrosurgical scalpel has been used to cut virtually every type of tissue; its use in division of muscle or other highly vascular tissue is generally accepted procedure. By using blended currents, muscular tissue can be divided with less blood loss and in less operating time. The small blood vessels traversing muscular tissue can be effectively coagulated without the necessity of using ligatures that are difficult to place unless one includes significant amounts of normal tissue. With electrotomy of muscular tissues, particular attention should be made to large vessels; they can be incompletely coagulated, may retract, and may form a hematoma. If muscle twitching is a problem, one should tense the muscle between one’s fingers to facilitate transection.
Although I do not routinely use them, electrosurgical scalpels and loops have been advocated for performing tonsillectomies, uvulectomies, ventriculocordectomies, anal sacculectomies, and skin tumor resections.
Electrocoagulation
The electrosurgical apparatus is extremely useful for coagulation of small bleeding vessels. A damped wave pattern provides the ultimate current for coagulation. Proper technique is required, and the technique of “frying tissue until it pops” is to be avoided. This practice is comparable to mass ligation of a bleeding point, and both lead to unnecessary tissue necrosis.
Vessels less than 1.5 mm in diameter can be sealed by pinpoint electrocoagulation. If larger vessels are coagulated by this method, delayed breakdown and hemorrhage may occur. Because fluids are current conductors, the field must be dry in the area surrounding the bleeding vessel. There are two ways to coagulate a bleeding vessel properly. The first is to apply the activated tip directly onto the vessel. The end point of coagulation is determined by tissue contraction and color change. A more precise method is to occlude the vessel initially with a hemostat or plain tissue thumb forceps. The active electrode is applied directly to the surgical instrument, which carries the current directly to the vessel. Care should be taken to prevent unwanted coagulation by not allowing the instrument to rest on normal tissue when the current is applied.
Electrofulguration and Electrodesiccation
These electrosurgical techniques cause dehydration and super- ficial destruction by a high-voltage, high-frequency current. These techniques are uniterminal; an indifferent electrode is not used. Electrofulguration damages tissue by electrical energy transmitted through an electrical arc or spark. Electrodesiccation is similar, although the electrode directly touches the lesion (See Figure 3-4). Tissue damage is deeper than with fulguration and may be difficult to control. Electrofulguration of perianal fistulas after a sharp “deroofing” procedure has produced encouraging results. Electrodesiccation has been used for removal of superficial skin lesions.
Precautions
Accidental burns are probably the most frequently observed complication of electrosurgery. It is imperative that an adequate indifferent electrode (“ground plate”) be incorporated in the system. Because of its large surface area, the indifferent electrode normally provides a low current density to complete the electrosurgical circuit. If contact between patient and plate is inadequate, however, high density electrical current can easily cause a burn (Figure 3-9). Although the indifferent electrode is designed to be the preferential pathway for the current, a faulty connection between the plate and the unit can result in a burn where the patient touches the metal operating table or the attachment sites of electrical monitoring equipment.
More expensive units have a 60 cycle monitoring current flowing through the “ground plate” system. A break in the ground wire or in its ground plate connection interrupts the monitoring current and sounds an alarm. Electrolyte jellies and a large area of contact with the patient are recommended to lower skin resistance and to provide more intimate contact between the skin and the indifferent electrode.
Explosions and fire are potential hazards when inflammable anesthetics, such as ether, chloroform, and cyclopropane, and inflammable skin preparations, such as alcohol, are used.
Electrical channeling occurs when the treatment electrode is used on tissue that has a thin connection to the body. An example is the testicle mobilized out of the scrotum. If electrocoagulation is used, electric energy will be channeled or funneled along the spermatic cord and will cause heat damage.
Cardiac pacemakers are implanted with increasing frequency in veterinary medicine, and the veterinary surgeon should be aware that high frequency electric energy may cause a cardiac arrest by interfering with the operation of the pacemaker.
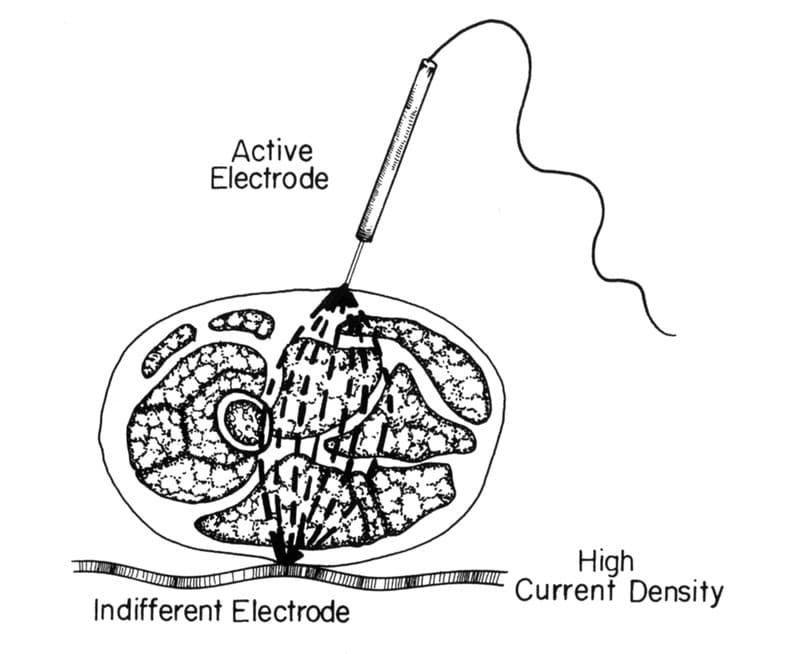
Figure 3-9. High current density produced at the indifferent electrode with improper technique.
Suggested Readings
- Battig CG. Electrosurgical burn injuries and their prevention. JAMA 1968;204:91.
- Fucci V, Elkins AD. Electrosurgery: principles and guidelines in veterinary medicine. Comp Contin Educ Pract Vet 1991;13:407.
- Giddard DW, Jones WR, Wescott JW. Electrosurgical units: particular attention to tube, spark gap and solid state generated currents–their differences and similarities. J Urol 1972;107: 1051.
- Glover JL, Bendick PJ, Link WJ. The use of thermal knives in surgery: electrosurgery, lasers, plasma scalpel. Curr Probl Surg 1978; 15:7.
- Greene JA, Knecht CD. Electrosurgery: a review. Vet Surg 1980;9:27.
- Greene JA, Knecht CD. Healing of sharp incisions and electroincisions in dogs: a comparative study. Vet Surg 1980;9:42.
- Ormrod AN. Electrosurgery: its usefulness and limitations for the small animal surgeon. Vet Rec 1963;75:1095.
- Swerdlow DB, et al. Electrosurgery: principles and use. Dis Colon Rectum 1974;17:482.
- Wald AS, Mazzia VDB, Spencer FC. Accidental burns associated with electrocautery. JAMA 1971;217:916.
Electrosurgery–Radiosurgery
A. D. Elkins
Introduction
Electrosurgical units are used to some degree in many veterinary practices. These units are often incorrectly used and in most hospitals under-utilized due to a lack of understanding of proper technique. The use of radiosurgery reduces operative time when used correctly with no delay in healing. The following discussion describes the difference in low frequency, electrosurgery and high frequency (3.8 to 4.0) radiosurgery units and provides a guideline for their proper use.
Radiosurgery is defined as the use of energy created by high frequency alternating current to perform surgical procedures. This is in contrast to electrosurgery in which low frequency (.5 mhz to 3.7 mhz) alternating current is used. The resistance of the tissue to the passage of this current creates heat internally in the tissue resulting in either cutting or coagulation.1 In radiosurgery, two electrodes (an active electrode and a patient return plate) of greatly different sizes resulting in increased current density at the point of the smaller active electrode are utilized. (Figure 3-10). While the electrode itself remains cold, the highly concentrated high frequency energy creates molecular heat inside each cell. The intercellular water boils and creates a microexplosion, thus incising tissue. The key to successful use of radiosurgery is control of the heat adjacent to the primary incision. By the choice of electrodes and selection and adjustment of the current, the surgeon controls the effect of this energy on the tissues to achieve the desired results. The ideal frequency for radiosugery is 3.8 to 4.0 MHz.2 This frequency allows for consistent primary healing of skin incisions. When low frequency energy is used to perform a skin incision, the risk of having delayed tissue healing increases due to the build up of lateral heat in the tissue.

Figure 3-10. Active electrode (wire) and indifferent plate.
A 4.0 mhz radiosurgery incision, unlike a scalpel blade incision, requires no pressure. The results are technique related (these techniques will be discussed later). Most of the factors related to a successful outcome are controlled by the surgeon. The buildup of lateral heat adjacent to an incision should be avoided. The following formula expresses the factors involved in the development of lateral heat.

The only factor not in the surgeon’s control is the output frequency of the equipment used. As can be seen from the above formula, the lower the frequency, the more lateral heat produced.3
Radiosurgery can be used for making an incision, excising a mass, obtaining a biopsy or controlling hemorrhage. The majority of veterinarians who use electrosurgical units use them primarily for hemorrhage control.
Electrocautery
The term electrocautery denotes the use of a hot iron to stop bleeding. The use of cautery to control hemorrhage dates back to the ancient Egyptains.1 Low voltage current is used to heat an electrode. When this heated electrode is applied to tissue a thermal burn occurs. The destructive effect on tissue is heat coagulation and hemorrhage control. Using electrocautery causes collateral damage to the tissue, resulting in delayed healing, therefore, electrocautery is not the ideal method of hemorrhage control. When describing the use of a radiosurgery unit to stop hemorrhage, the correct term is electrocoagulation. Since there is no heat build-up at the electrode tip this is not cautery. The terms electrocautery and electrocoagulation have been incorrectly used synonymously in the literature.
Electrocoagulation
Electrocoagulation is the use of electrosurgical current to control hemorrhage. Vessels up to 2 mms in diameter can be coagulated with electrosurgery units. Vessels larger than 2 mms should be ligated. Utilizing proper technique by touching an electrode to a vessel in a relatively dry field or to a hemostat which has been applied to the vessel will form a coagulum at the end of a vessel. Excessive heating of the tissue until it snaps or pops should be avoided as this causes increased tissue necrosis. The use of electrocoagulation to control hemorrhage results in better visibility thus allowing the surgeon to be more efficient and reduce operative time. It also reduces the amount of foreign material left in a wound from ligatures. The majority of surgical procedures can benefit from the use of radiosurgical electrocoagulation. It has been said that a poor surgeon is not made better by the use of radiosurgery, only more efficient.
The application of an electrode to an actively bleeding vessel is only successful in controlling hemorrhage if the bleeding is temporarily arrested. This can be accomplished by either direct pressure to the vessel then applying the electrode or clamping a hemostat to the vessel then touching the electrode to the hemostat (Figure 3-11).
When touching the electrode directly to the vessel, a larger electrode, like a ball or blade, is more effective (Figure 3-12). Either of these techniques is effective if the field is relatively dry. This is known as monopolar electrocoagulation. An alternative is the use of biopolar forceps. (Figure 3-13). In using bipolar forceps, one tip acts as the active electrode and the other the indifferent plate. This gives precise pinpoint control of the electrocoagulation effect. It can be used anywhere in the body, but is very useful near delicate and sensitive tissue such as the spinal cord, eye, nerves, or large vessels. Bipolar forceps are very useful for surgery in avian and small exotic species.

Figure 3-11. Thumb forceps on vessel with electrode applied to thumb forceps.

Figure 3-12. Ball electrode and blade electrode used for electrocogulation.

Figure 3-13. Bipolar forces.
Electroincision
An incision with high frequency radiosurgery may replace a scalpel incision in any tissue. This being said, it is imperative to use proper technique and a frequency of 3.8 to 4.0 MHz when making skin incisions. A frequency lower than 3.8 to 4.0 MHz risks the buildup of lateral heat in the tissue. This may result in delayed healing and/or dehiscence of the incision.4
Four wave forms or current types may be selected when using a high frequency radiosurgery unit. These wave forms are:
A. Fully filtered or continuous wave form is a continuous high frequency waveform that produces a smooth cut (Figure 3-14). It gives a 90% cut and a 10% coagulation effect. It generates the least amount of lateral heat. When this waveform is delivered by a fine wire electrode, it is comparable to a scalpel blade with excellent healing properties4 (Figure 3-15). A biopsy obtained with this waveform creates a micro-smooth cut with no heat artifact at the edges. This allows an accurate reading by the pathologist on the biopsy specimen. The fully filtered/ continuous waveform should always be used when making skin incisions.
B. Fully rectified waveform is not as smooth as the continuous wave form; thus reducing the efficiency of the cut (Figure 3-16). It does, however, achieve a significant amount of hemostasis. When using a unit with 3.8 to 4.0 output frequency, minimal thermal damage can be expected. This setting produces a 50% cut and 50% coagulation effect. It is ideal for sub-cutaneous tissue incision, dissection or when working in vascular tissue such as the oral cavity.
C. Partially rectified waveform is an intermittent transmission of high frequency waves that increases lateral heat production (Figure 3-17). This is ideal for electrocagulation of small vessels up to 2 mms. It gives 90% coagulation with a 10% cut effect.
D. Fulguration is a spark-gap wave form (Figure 3-18). Fulguration rapidly dehydrates or desiccates tissue. This is ideal for areas where the surgeon wants intentional tissue destruction (such as perianal fistula, abscess or draining tracts). This may also be used with a ball electrode to control diffuse, weeping type bleeding. The tissue destruction is self-limiting by the insulating effect of tissue carbonization, therefore only a superficial layer of tissue is damaged.

Figure 3-14. Oscilloscope showing fully filtered, 90% cut waveform. Notice the smooth, continuous nature of the waveform

Figure 3-15. Fine Wire electrode.

Figure 3-16. Fully recitifed, 50% cut, 50% coagulation waveform on oscilloscope.

Figure 3-17. Partially recitifed, 90% coagulation/10% cut waveform on oscilloscope.
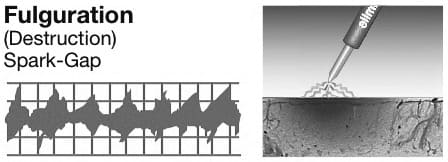
Figure 3-18. Fulguration waveform on oscilloscope.
Factors to Consider in Selecting
Electrosurgery
Tissue selectability is determined by the degree fibers are cut compared with how much they shift as energy is applied.4 This is important in making incisions around the eye or other mobile skin areas. When incising skin in these areas with a scalpel blade, significant pressure is required and the final incision may not have the desired appearance. This is avoided with radiosurgery in that it is a pressureless cut. Pre-planning the incision by drawing its margins with a skin marker may be helpful.
Multiple studies have been performed comparing high frequency radiosurgery, scalpel and carbon dioxide laser.5 In one study in human oviduct excision, it was found that radiosurgery produced less lateral heat damage to the surrounding tissue than laser.5 Although the learning curve with radiosurgery is not steep, poor technique using this method of tissue incision may result in delayed wound healing.
The following points should be considered when utilizing radiosurgery:
A. Use a high frequency (3.8 to 4.0 MHz) unit when making skin incisions. This helps prevent lateral heat damage.
B. Chose the smallest wire electrode available to reduce tissue resistance and heat build-up.
C. Use the full filtered or continuous wave form when making skin incisions.
D. Use the lowest power setting possible without producing drag of the electrode through the tissue. The electrode should pass through tissue effortlessly with minimal sparking or plume production. There should be minimal to no charring of the tissue.
E. Electrode contact time with the tissue is directly proportional to the lateral heat transferred to the tissue. The electrode should be moved rapidly through the tissue. If you have to return to the same area, allow an eight second lag period to occur. This allows heat build-up in the tissue to dissipate.
F. Avoid contact of the electrode with cartilage, bone or enamel. The most sensitive tissue is cartilage due to its high water content. Therefore, when performing a procedure like a feline onychectomy the distal portion of P2 should be avoided.
Precautions
Accidental burns to the patient are the most serious observed complication to electrosurgery.4 Many electrosurgery units utilize a metal ground plate. If good contact between the ground plate and patient is not present, a burn can be created. The ground plate is designed to be the deferential preferred pathway for current. If a faulty connection exits then a burn can occur.1 Electrolyte jelly and a large area of contact with the patient are recommended to lower skin resistance and to provide more intimate contact between the skin and the ground plate.4
A safer system is the use of an indifferent plate or an antenna plate found with the the Ellman Surgitron or Dual Frequency unita (Figure 3-19). This is a plastic coated plate that requires no conductive gel and does not have to be in contact with the patient. This indifferent plate can be placed under the surgical drape but it should be in close vicinity to the surgical site. This makes the unit more efficient and allows the surgeon to use a lower power setting.
Explosions or fire are potential hazards if using flammable liquids like alcohol. If alcohol is used in the skin preparation for surgery, allow an adequate time for the alcohol to dry.
In summary, this author has been performing radiosurgery with either an Ellman Surgitron (3.8 mhz) or the newer Dual Frequency (4.0 mhz) Unit for over 30 years. Excellent clinical results can be achieved when high frequency, low temperature radiofrequency devices are used and good radiosurgery principles are followed. The modern radiowave units are affordable, durable and become work horses in surgical practice. Some form of radiosurgery, either for making an incision, excision, dissection or hemostasis is used on each surgery performed.
References
- Parker RB: Electrosurgery and Laser Surgery in Bojrab MJ, ed; Current Techniques in Small Animal Surgery. Philadelphia: Lea & Febiger, P. 41.
- Fucci V, Elkins AD: Electrosurgery: Principles and Guidelines in Veterinary Medicine. Comp Contin Educ Pract Vet 1991; 13; 407.
- Miller WM: Using High-Frequency Radiowave Technology in Veterinary Surgery. Vet Med Sept 2004; 796-802.
- Olivar AC et al: Transmission Electron Microscopy: Evaluation of Damage in Human Oviducts Caused by Different Surgical Instrumetns, Ann Clin Lab Sci. 1999 29 (4): 281-285.
Lasers in Veterinary Medicine – An Introduction to Surgical Lasers
Kenneth E. Bartels
Introduction
The principles necessary for the concept of laser development were reported as early as the 19th century with Bohr’s theory of optical resonance. In 1917, Einstein proposed the concept of stimulated light emission. Finally, in 1960, Theodore Maiman developed the first laser which was a pulsed ruby laser.1 Since medical use began in the early 1960’s, the laser has been considered by many to be “a tool in search of an application.” Many of the earlier medical lasers were extremely cumbersome, expensive, and difficult to maintain. However, as biomedical laser technology merged with military and industrial efforts, innovations and improvements in devices and development of new concepts occurred and continue today. Developmental requirements to implement these new technologies include improvements in light delivery systems (robust articulated arms, small diameter wave-guides, and small-diameter optical fibers), compatible laser wavelengths, endoscopic visualization, and more portable, economical, user-friendly biomedical lasers.
Unique Properties of a Laser
Light bulbs and lasers both generate light, which is the common name for electromagnetic energy that we can see. The electromagnetic spectrum extends from the very short wavelengths (gamma radiation at 10-11 m) to radio waves (10-1). Laser wavelengths fall between the infrared and ultraviolet wavelengths of electromagnetic radiation, which include the invisible and visible light spectrum. The word “LASER” is an acronym that stands for Light Amplification by the Stimulated Emission of Radiation. An extensive discussion in laser physics is not consistent with this general overview. In simpler terms, as a bow stores energy and releases it to propel an arrow, a laser stores energy in atoms, concentrates it, and then releases it in powerful waves of light energy. This process is called stimulated emission. The resulting emission of photons resonates between mirrored ends of a laser resonating cavity. These bouncing photons further excite other atoms in a laser medium. Momentum builds until a highly concentrated beam of light passes through a partially transmissive mirror at one end of the laser resonating cavity.2
Like sound through air or water on a lake, light travels in waves. Moreover, the color of light is governed by its frequency and wavelength (distance of one peak to the next). Normal white light is incoherent and includes many wavelengths radiating in all directions. The peaks and valleys of the waves do not coincide. A prism illustrates this as it sorts a white light into individual colors of the rainbow. Laser light does differ from ordinary light much as music does from plain noise. Laser light, in comparison to ordinary light, is coherent. Each peak and valley of individual light waves align exactly. If laser light waves could be heard, their sound would resonate with the clarity of a single musical tone. In addition, laser light is of one wavelength (one color), or is monochromatic. Finally, laser light is collimated, or non-divergent, and directional. Parallel light waves move in unison, reinforcing each other as they travel through space forming a virtual tidal wave of laser energy.
Today’s technology allows the manufacture of lasers that produce wavelengths of light extending from ultraviolet to far-infrared wavelengths. Devices range in size from miniaturized diode lasers capable of being passed through the eye of a needle to a free electron laser which covers the entire length of a large building. However, each laser is composed of the same basic components and functions according to the lasing medium stimulated to produce energy emission and light. Please refer to Figure 3-20: Laser Components.
Laser wavelength refers to the physical distance between crests of successive waves in the laser beam, indicated in units of length expressed as nanometers or microns. By definition, 1 nanometer (nm) = 109 meter, or one-billionth of a meter. One micron (μm) is equal to 106 meter or 1000 nm. More common medical lasers include ultraviolet (193 nm and 308 nm), visible (532 nm and 630 nm), near-infrared (805 nm, 980 nm, and 1064 nm), mid-infrared (2100 nm), and far-infrared (10,600 nm) wavelength systems. This means that many of the common laser wavelengths used for medical applications (diode/805-980 nm; carbon dioxide/10,600 nm) cannot be seen by the human eye and can be extremely dangerous as far as ocular hazards due to this fact.2

Figure 3-20. Components of a laser.
Types of Laser-Tissue Interaction and Laser Operational Modes
Laser radiation must be converted into another form of energy to produce a therapeutic effect. Laser-tissue interactions are categorized according to whether laser energy is converted into heat (photothermal), chemical energy (photochemical), or acoustic (photomechanical/photodisruptive) energy. Photothermal interactions occur when laser light is absorbed by tissue and converted into thermal energy, which results in a rise in tissue temperature. When far-infrared laser wavelengths (10,600 nm) are used, the water component of tissue plays a predominant role in the absorption of laser energy. Water is heated directly with laser energy, and other molecules may then be indirectly heated via heat conduction. Other tissue components (hemoglobin, melanin, proteins) may also absorb energy at specific mid-infrared wavelengths (805, 980, 1064 nm) and play an important role in the tissue heating process. The absorption of laser energy in any tissue is the sum of the absorptions of each of the tissue components coupled with the absorption coefficient of water. For example, the effective absorption depth or extinction coefficient of CO2 carbon dioxide laser energy (10,600 nm), which is heavily absorbed by water, is approximately 0.030 mm, but is about 1 to 3 mm for the diode (805/980 nm) or neodymium yttrium aluminum garnet Nd:YAG (1064 nm) lasers, which are less heavily absorbed by water.3
Visible laser wavelengths (400 to 700 nm) are poorly absorbed by water and usually rely on blood or other endogenous tissue pigments or exogenous photoactive compounds to absorb laser light and convert them to heat or active photochemical components. Naturally occurring molecules that absorb visible wavelengths include hemoglobin and melanin. Protein molecules, DNA, and RNA absorb ultraviolet wavelengths strongly and usually play a dominant role in converting UV light energy into heat. Figure 3-21 illustrates the water absorption curve, which is an essential component in understanding the concept of laser-tissue interaction.3
Pulsed laser energy generated by the dye, holmium, or erbium lasers can be converted into acoustic (photomechanical) energy in the form of a shock wave or a high-pressure wave, which can physically disrupt the targeted structure when combined with a photothermal interaction (laser lithotripsy). Laser light can also be absorbed and converted into chemical energy (photochemical) that can break chemical bonds directly or excite molecules into a biochemically reactive state. Laser wavelength is the critical factor in this process. Short ultraviolet wavelengths (e.g., 193 nm) are needed to maximize chemical bond-breaking processes while minimizing the photothermal process as observed with excimer laser energy commonly used in human ophthalmologic procedures (LASIK). 2,3

Figure 3-21. Laser tissue optics: water absorption curve. This graph illustrates the varying degrees of absorption of a specific wavelength (color) of light by water compared to absorption in oxyhemoglobin, melanin, and tissue proteins including amino acids, DNA, and RNA. Ar, argon; KTP, potassium titanyl phosphate; XeCI, xenon chloride; YAG, yttrium aluminum garnet.
Specific visible wavelengths (630 to 730 nm) can also induce photobiochemical reactions. This type of reaction can be related to photodynamic laser interaction. In general, photodynamic interactions employ light-absorbing molecules (photosensitizers such as hematoporphyrin derivatives) to produce a biochemically reactive form of oxygen (singlet oxygen) in tissue when activated by light of a specific wavelength. Photodynamic interactions are considered to be a special type of photochemical interaction. The therapeutic process is called photodynamic therapy (PDT).2,4,5
Biostimulation is a process induced by lower power lasers (5 mW to 12 W/635 to 1064 nm) that may provide pain relief, stimulate wound healing, or alter other biological processes. The entire concept is considered controversial due partly to the fact that all of the physical, biochemical, and physiologic mechanisms are not well understood. Many of the reported results are mostly subjective in nature and are difficult to quantify. However, this therapeutic modality may gain favor as more objective studies are reported.5,6
Laser light focused on tissue may be reflected, absorbed, scattered throughout, or transmitted through the tissue. The application of laser energy is very dependent on wavelength, as mentioned previously. It is also essential to say the effect of a laser on tissue is dependent on power. Power is usually expressed in watts. When time is figured into the equation of energy delivery, the term “joule” is used, which is defined as a watt/second. Focal spot size (size of the incident beam of the laser light) results in the concentration of energy within an area, known as “power density” and expressed as watts/cm2. The advantage of a small spot size is that laser energy is more concentrated and causes less collateral damage, where fewer cells will be affected and destroyed at the margins of an incision. When a rapid, deep incision is required, a small spot size is advantageous in that it will concentrate a high amount of energy into the tissue leading to rapid vaporization. A larger spot size will be less precise and enhance tissue coagulation rather than vaporization. The important term “fluency” takes into account the “time domain” or laser “on time” and is used to describe the total energy delivered to the target tissue in joules/cm2. Total energy delivered to the tissue target is extremely important when considering a laser beam that is set for a pulsed mode delivery.2,7
Biomedical lasers can operate in continuous wave (CW) or pulse mode (single pulse, chopped or repeat, and super-pulse). Laser output in CW mode remains constant, whereas lasers operating in pulse mode deliver short bursts of energy. Manipulating pulse duration and pulse frequency allows the surgeon to adapt laser output to suit a particular clinical application, as well as ensure exquisite control. A laser operating in single pulse mode emits a single, user-defined pulse of energy lasting from a few milli-seconds to several seconds. When operating in chopped or gated mode, a laser emits energy at selected pulse duration and frequency. The primary difference between chopped and CW emission is that chopped mode has periodic gaps of zero power in an otherwise CW emission.2,7
Superpulse is another temporal mode of CO2 laser energy delivery that incorporates high peak power in short, high frequency pulses. Lasers operating in a super-pulse mode deliver extremely high peak power, often 7-10 times higher than the CW maximum power, short pulse duration, and shorter off time than chopped mode. The maximum peak power in super-pulse mode is higher than the maximum CW power by a factor that depends on type of laser and its specific design. The main advantage of using a carbon dioxide laser in superpulse mode is the reduction of carbon formation or a decrease in char.2,7
In very simple terms, a volume of tissue cools between rapid pulses of targeted energy, a phenomenon known as thermal relaxation. When laser exposure (pulse duration) is less than thermal relaxation time for the targeted structures, maximal thermal confinement occurs and vaporization (ablation) occurs without damage to non-targeted collateral structures. This concept along with minimal carbon formation on the target tissue surface provides the laser surgeon with exquisite control and precise vaporization not seen with other means of tissue dissection. For surface ablation, use of computerized micro-processors, accessories for some high power carbon dioxide lasers, utilize superpulse laser energy delivery coupled with optomechanical hand-pieces to decrease the “dwell time” a laser beam interacts with the tissue surface. These scanning devices decrease surface carbonization and permit rapid and precise laser vaporization.3,7,8
Pulsed laser energy can be converted into photomechanical (photo acoustic) or photothermal energy, depending upon pulse duration, peak power density, and pulse frequency. Photomechanical effects occur when very short (nanosecond – 10-9 sec.), high-power laser energy pulses are directed at tissue through a small-diameter optical fiber. The energy plasma-induced shock waves generated at the tip of the optical fiber mechanically disrupts the targeted tissue or calculi. Photomechanical interactions are important in many specialized laser applications, including lithotripsy and ophthalmologic surgery.9,10
Photodisruption is a relatively new term used to designate tissue interaction related to effects of ultrafast (femtosecond – 10-15 sec.) laser pulses. Laser light is tightly focused to tremendous power density levels (1012 W/cm2) but pulse energies of only 1 uJ. The photomechanical and photothermal side effects are negligible. Tissue is ionized and optically broken down by a process called multiphoton absorption and offers the possibilities to perform very precise surgical operations at the cellular and sub-cellular levels.9
The tissue response to the application of photothermal laser energy is a very dynamic process. Changes in the local microcirculation influence the tissue reaction to additional laser energy. When the beam interacts with tissue, the photothermal effect produces a characteristic lesion in living tissue. Initially, hyperthermia and desiccation of tissue and cells occurs and then are followed by coagulation and vaporization. At the impact site, a crater may be formed when tissue has been vaporized from the region. Immediately surrounding the cavity is an area of hyperthermia, cellular coagulation, and eventually, necrosis. This zone is created by the diffusion of laser energy from the point of laser impact. Immediately adjacent to this zone is an area of cellular edema without evidence of alteration in the collagen stroma. The milder thermal injury to the tissue in this region may resolve within 48-72 hours. These phenomena are illustrated in figure 3-22. The generation of smoke, hemorrhage, and char can interfere with the incident laser beam by resulting in scatter, reflection, and absorption of the laser energy and may result in uncontrolled effects on the target tissue or adjacent structures.3,7,10
Precise control of hemorrhage and inflammation by photothermal sealing of blood vessels, lymphatic vessels, and incised nerve endings is perceived by most to be distinct advantages of laser surgery. These benefits relate directly to laser tissue interaction depending on wavelength, power, and fluency. However, by inhibiting the early stages of the inflammatory process (lag phase) due to cellular constituents and platelets not being immediately available at the wound site, the healing of laser incisions is minimally delayed. Laser incisions, discounting collateral photothermal effects due to poor surgical technique, gain strength as quickly as incisions made by a steel scalpel and incisional tensile strengths are comparable within 10 to 14 days.11,12
Laser vaporization is the process of removing solid tissue by converting it into a gaseous vapor or plume. This is usually in the form of steam or smoke, but laser plume may also contain noxious substances. Therefore, the use of smoke evacuation during laser surgery is deemed essential. Safety issues will be discussed more specifically in a following section. The term “vaporization” is used as a synonym for tissue ablation.
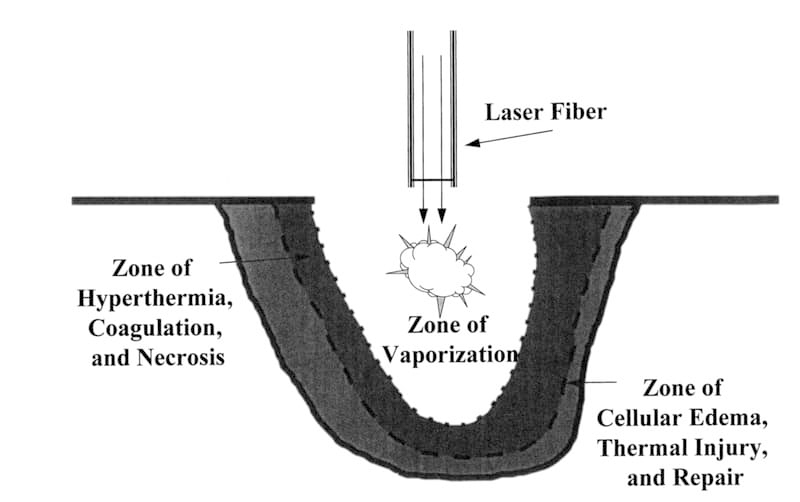
Figure 3-22. Laser tissue interaction. The generalized tissue response to the application of laser energy results in zones of vaporization, necrosis, and reversible thermal injury.
Types of Commonly used Medical Lasers
The development and use of biomedical lasers is considered to be a significant step ahead of mechanical instruments, but falls short of what is needed to be considered as the optimal “light knife” for every surgical situation. Considering differences in laser-tissue interaction, it’s still very uncertain whether an “ideal” laser wavelength will ever exist. Discounting future use of free-electron lasers with multi-wavelength variability, acceptance of biomedical use of lasers with a fixed-wavelength has depended more on cost, capability for fiberoptic delivery, portability, flexibility, ease of use, and dependability. 2,4,13
In medicine today, many different types of biomedical lasers are in use. Each instrument is usually acquired for a specific purpose in mind, such as dermatologic or endoscopic applications. Overall, the use of laser energy can be an extremely precise and controlled method for tissue removal or cellular destruction. Medical lasers are expensive and require a dedication to proper use and objective evaluation. Lasers in common use today are the carbon dioxide (CO2), neodymium yttrium aluminum garnet (Nd: YAG), diode, holmium: YAG (Ho: YAG), and dye lasers. The following general descriptions are meant to be used as an overall guide to medical lasers. In no way should it be considered complete. Changes in laser types, wavelength preference, and delivery devices are made on a frequent basis, since they are closely aligned with changes in today’s technologic advancements in computer hardware and software.
Carbon Dioxide Laser (CO -10,600 nm)
The carbon dioxide laser was one of the first medical lasers used for tissue ablation. At 10,600 nm, the wavelength is ideal for cutting and vaporization because it is highly absorbed by water. It can cut tissue cleanly when the beam is focused onto tissue and can debulk tissue by photovaporization when defocused. Because of the high absorption the 10,600 nm wavelength in water, CO2 laser energy transmission requires energy delivery through a series of mirrors in an articulated arm or through a semi-rigid waveguide, which makes it awkward for use in an open abdomen or in other localized and confined areas. However, thermal injury from a given amount of energy is relatively superficial (50 to 100 μm in depth).2 The net surgical result is expressed as “What you see is what you get!” when using the carbon dioxide laser. The learning curve for using a carbon dioxide laser seems to be shorter than with other surgical laser wavelengths (805, 980, 1064 nm) which are optically scattered more in tissue. However, since CO2 laser delivery systems (articulated arms, hollow waveguides) must be used in a non-contact mode, the tactile appreciation for tissue is lost. This is a disadvantage which can be overcome quite easily with practice. Pertinent engineering specifications for carbon dioxide lasers include the “excitation” mechanism. That is, how the CO2 gas mixture in the resonating cavity is stimulated to produce 10,600 nm light. Direct current (DC) devices are usually larger machines capable of emitting higher power (> 20 W). Most of these devices use a water cooling mechanism that is either closed or can be connected to a circulating cooling water system. Radiofrequency (RF) excited CO2 lasers are usually smaller, more robust devices that are either cooled by convection or by an integral cooling fan. RF excited devices usually emit lower power laser energy (< 20 W). 10,14
Nd: YAG Laser (Neodymium Yttrium Aluminum Garnet-1064 nm)
The Nd: YAG or “YAG” laser differs from the CO2 laser because the wavelength allows transmittance though tissue in addition to surface absorption. High powers up to 100 watts can be delivered through small-core optical fibers that can easily be inserted through the accessory channels of standard GI endoscopes. Since the Nd:YAG laser has less specific absorption by water and hemoglobin than the carbon dioxide laser, the depth of thermal injury can exceed 3 mm in most tissues, which can be useful for coagulation of large volumes of tissue. Fairly rapid tissue vaporization in non-contact mode is possible with a bare non-contact fiber, but collateral thermal injury may be substantial. Power levels approaching at least 50 watts are usually needed for these soft tissue applications. 2
Continuous wave (CW) Nd: YAG and diode lasers can be used with “hot-tip” delivery systems to perform vaporization and cutting of soft tissue in a contact mode with surgical precision, little collateral thermal injury, and good hemostasis. Hot-tip fibers include sculpted quartz fibers, contact-tipped sapphire fibers, metal-capped fibers, temperature controlled bare fibers, and dual effect fibers. In principle, contact use of fibers for mechanical coaptation of tissue while it is being heated can be advantageous for hemostasis and controlled excision. Use of contact tips for endoscopic application is widely accepted, but some tips are too large to insert through flexible endoscopes.15,16,17
Diode Laser (635, 805, 980 nm)
Advancement of semiconductor diode laser development has progressed tremendously in concert with other aspects of medicine described previously. Engineering and commercial specifications have allowed development of devices with wavelengths varying from approximately 635 to 980 nm. Newer technologies may actually allow evolution of diode lasers capable of emitting wavelengths in the mid-infrared range (1.9 to 2.1 μm). 2
Therapeutic products that employ semiconductor diode lasers were first approved for surgical use in this country in 1989. Diode lasers (1 to 4 watts) are also used for photocoagulation of retinal and other ocular tissues, and have been employed for ophthalmologic applications since approximately 1984.18 The compact size and high efficiency offer significant ergonomic and economic advantages. High power semiconductor diode lasers appropriate for other surgical applications have been recently introduced for a variety of uses. These lasers currently provide up to 25 to 100 watts at 805 nm or 980 nm, wavelengths that can penetrate deeply into most types of soft tissue, and produce tissue interactions comparable to the Nd: YAG laser (1064 nm).15 The theoretical difference between use of a diode laser at 805 nm and one emitting a 980 nm wavelength is that a 980 nm device is absorbed to a greater extent by water than is the 805 nm laser, but in actual clinical practice this difference is negligible. Diode lasers can be used with bare-fiber delivery accessories in non-contact mode for deep coagulation, or with hot-tip fibers for precise cutting or vaporization in contact mode. As mentioned, diode lasers can be used for many of the same applications as 1064 nm continuous wave Nd: YAG lasers. However, surgical diode lasers offer considerable advantages compared to Nd: YAG lasers. They are smaller, lighter, require less maintenance, are extremely user-friendly, and can be more economical. Some medical device manufacturers predict prices for diode lasers will eventually drop to the point where they may be competitive with high-end electrosurgical equipment.
Additional applications for diode laser energy have been for chromophore enhanced tissue ablation or coagulation, tissue fusion or laser welding, and photodynamic therapy. The use of sutureless tissue repair employing laser energy has emerged over the last decade. Tissue welding or fusion has the potential to be one of the most important technical developments in surgery. Used in conjunction with laparoscopic as well as open procedures, laser energy used with biological glue or “solder” reinforcement can provide a higher leakage pressure for vascular and alimentary tract structures than sutures alone. Preliminary investigations involving selective fusion of nerves, urethral tissue, skin, tracheal mucosa, and even bone fragments have also shown promise. Despite a decade of laboratory success in which the superiority of laser tissue welding has been demonstrated, there is still not much clinical use of this technology.13
Diode laser (805 nm) induced photothermolysis of tissue selectively stained with indocyanine green (ICG) has shown promise for selective coagulation/vaporization of tumors and contaminated wounds.4 Diode laser wavelengths of 805 nm have also been reported as being used for tissue welding investigations because applications have been centered around the peak absorption spectrum of indocyanine green (780-820 nm), the selective chromophore used in fibrinogen solder. Laser energy required for tissue fusion is significantly lower (300 mW to 9.6 W/ cm2) than for incisional/ablative procedures, since minimal thermal changes are required to produce noncovalent bonding between denatured collagen strands and produce the weld.9 The small, convenient size coupled with reliability and user friendliness has also focused extensive diode laser development for applications in photodynamic therapy, primarily at 635 nm wavelength.19
Ho: YAG Laser
(Holmium Yttrium Aluminum Garnet-2100 nm)
Clinical holmium lasers have appeared in recent years for arthroscopic surgery, general surgery, laser angioplasty, and thermal sclerostomy. Additional applications have been implemented for laser diskectomy, removal of sessile polyps in the gastrointestinal tract, and otorhinolaryngeal procedures. The main attraction of the holmium laser is its ability to cut and vaporize soft tissue like a carbon dioxide laser, with the added advantage that holmium energy can be delivered through flexible, low OH, quartz optical fibers. Good surgical precision and control can be obtained with a bare optical fiber. Unlike visible wavelength lasers, and again similar to the carbon dioxide laser, photothermal interactions with the holmium laser do not rely on hemoglobin or other pigments for efficient heating of tissue. The water component of tissue is responsible for absorbing holmium laser energy (2100 nm) and converting it to heat. The depth of absorption is quite shallow at approximately 0.3 mm. When cutting or vaporizing tissue, actual zones of thermal injury vary from 0.1 to 1 mm, depending on exposure parameters and the type of tissue. These small thermal necrosis zones provide better surgical precision and adequate hemostasis.2 Current holmium instruments are flashlamp-pumped systems. The active laser medium consists of a chromium-sensitized yttrium aluminum garnet host crystal doped with holmium and thulium ions. This active medium is referred to as Thulium (Tm), Holmium (Ho), Chromium (Cr): YAG or THC: YAG, and is common to all holmium laser medical devices. Unlike the carbon dioxide laser, higher power holmium lasers cannot operate in a continuous wave mode at room temperature. The relatively low pulse rates (10 to 20 Hz) available from most holmium lasers may be considered as a disadvantage since cutting may be slow or result in jagged tissue edges during incisional applications. In addition, at higher pulse energies (> 1 Joule), considerable amounts of acoustical or mechanical energy are generated in tissue. An audible acoustical “pop” may be generated and actually heard during laser application. However, acoustical energy may be considered an advantage when using holmium energy for photodisruptive procedures such as lithotripsy of gallstones or urologic calculi. 20,21,22
Dye Laser (635 to 700 nm)
Pulsed and continuous wave dye lasers employ an active laser medium that consists of an organic dye dissolved in an appropriate solvent. For the dye laser to work, the dye solution must be re-circulated at high velocity through the laser resonator. Dye lasers are useful for medical applications because they can generate high output powers and pulse energy at wavelengths throughout the visible wavelength spectrum (400 to 700 nm). They are usually pumped by argon lasers, flashlamps, or a frequency-doubled YAG laser. Dye lasers have been used for lithotripsy of biliary and urologic calculi (504 nm-pulsed), activating photosensitizers for photodynamic therapy (635 to 720 nm CW), ophthalmologic operations (805 nm pulsed or CW), and dermatologic applications (577 to 585 nm pulsed) including treatment of birthmarks and removal of tattoos.2,5,13,20,23
Laser Delivery Systems
A delivery system refers to the optical hardware needed to transfer energy from the laser to the treatment site. Devices for guiding laser beams to the patient include articulated arms with internal mirrors, hollow waveguides, and optical fibers. Articulated arms and hollow waveguides are used with laser wavelengths (2800 nm to 10,600 nm) that cannot be transmitted through conventional fiber optics due to their light absorption characteristics. Laser energy delivery through an articulated arm has inherent disadvantages due to the size of the arm, durability, and its inability to be used for minimally invasive (endoscopic) procedures. Using carbon dioxide lasers with an articulated arm allows delivery of a precise collimated (Gaussian) focused beam to the incision site. Using a semi-rigid hollow wave-guide provides a non-collimated beam that is multi-model (top-hat) in nature, but still very precise since the laser energy is concentrated and directed through small, aperture delivery tips (0.2 to 1.4 mm diameter) that can be used for precise incisional and ablative applications. Hollow waveguides are advantageous in permitting greater flexibility for performing laser procedures but are not as useful as conventional fiber optic delivery through quartz fibers. Future advances in laser and optical waveguide technologies will include smaller diameter waveguides that can deliver collimated laser energy and be used through endoscopic portals for minimally invasive procedures. 2,16
The availability of functional and inexpensive optical fibers for laser delivery has played a crucial part in the acceptance of lasers for medical applications. The fibers used in laser medical delivery are made of quartz glass and have diameters ranging from 0.1 to 1 mm. Laser energy is transmitted and reflected along the bends and curves of the fiber until it reaches the tip where it exits.
The ability to transmit visible and near-infrared laser energy, small diameter and flexibility, lower cost, and ruggedness makes quartz optical fibers essential for endoscopic and other minimally invasive applications. Configurations of fiber tips (e.g., flat or cleaved, sculpted orb, chisel) and their ability to transmit energy is a physical science in its own right, but delivery parameters are primarily based on two factors, contact mode of delivery or non-contact mode of delivery. In non-contact mode, a free beam of focused laser energy is delivered to the tissue target surface. The power density and fluency of the laser beam determine the degree of photothermal interaction. Non-contact mode usually increases the surface area covered by laser energy which can decrease the power density and consequently decreases vaporization efficiency unless laser power output is increased. In contact mode, a laser optical fiber tip is brought into direct contact with the tissue target and the resulting photothermal interaction causes carbonization of the tip, which then becomes a focused “hot knife.” The chemical structure of certain optical fibers permits transmission of mid-infrared laser energy (Ho: YAG at 2100 nm through a low-OH polyamide fiber) and allows minimally invasive laser surgery through small diameter endoscopes and myelographic needles.16,24,25
Laser Safety
Even though sci-fi movies and television portray lasers as “death rays” and “phaser disintegrators,” the instrument is probably safer to use than a scalpel or scissors in the hands of a trained operator. However, lasers use by untrained individuals can be dangerous for both the operating team and the patient. Safety standards for medical laser applications have been issued that consider potential hazards and their control measures. The current consensus standard in the United States is through the American National Standards Institute’s (ANSI Z136.3) document entitled Safe Use of Lasers in Health Care (Available from Laser Institute of America, 13501 Ingenuity Drive, Suite 128, Orlando, FL 32826). Application of surgical lasers in veterinary medicine should adhere to these regulations and guidelines to ensure operator and patient safety. Laser hazards depend on the laser wavelength and power, the environment, and the personnel involved with the laser operation. The laser hazard is defined by a hazard classification (1 to 4). Surgical lasers are almost all classified as Class 4 laser products because they may represent a significant fire or skin hazard and also produce hazardous diffuse reflections. Hazardous diffuse reflections are of concern because the probability of damaging retinal exposure is extreme without proper eye protection. 26,27
With the biomedical application of lasers, the following safety concerns must be considered:
1. Inhalation of Smoke or Laser Plume
Laser surgery usually creates more smoke than electrosurgical procedures. Reports have mentioned that smoke products from lasers are really no different than those created by electrosurgery, although the quantity is greater. Some studies have actually isolated viable tumors cells from smoke evacuation tubes, so the concept of uncontrolled viral or bacterial vaporization must also be taken into account. Since even sterile smoke can be an irritant, all products of combustion as a result of laser vaporization must be evacuated with a dedicated smoke evacuator. The filters and tubes on these devices require maintenance and periodic replacement, increasing the cost of laser surgical procedures.
2. Laser Induced Combustion
Laser beams can cause fires. The obvious way to prevent laser induced combustion is to make certain the beam is always directed towards the surgery site. In addition, the use of moistened sponges surrounding the surgical site decreases the chance for accidental ignition of drapes, etc., especially when using wavelengths highly absorbed by water, such as the carbon dioxide laser. Polyvinyl chloride endotracheal tubes are especially prone to ignition. An endotracheal tube which is carrying oxygen will literally become an airway blowtorch instantaneously after impact of the laser beam. In airway and oral surgery, the endotracheal tube should be of a type that includes specific laser-safe tubes and less desirably, endotracheal tubes made of red-rubber protected by an application of reflective metal tape.
3. Eye and Skin Burns
Laser energy burns to the eyes or skin on the patient, operator, and assistants are of extreme importance for consideration. Safety glasses or goggles, specified for each laser wavelength, must be worn for every laser procedure. Saline moistened surgical sponges or even laser safety eyewear should be considered for protecting patient’s eyes. In addition, window barriers, laser safety warning lights, ebonized or a dulled, satin-type finish on surgical instruments to reduce reflection, and laser warning signs on doors are important safety aspects that should not be ignored. The potential for accidental burns and fires usually is related to accidental depression of the footswitch for the laser. All machines are equipped with a standby mode of operation in which the machine is running but laser energy cannot be activated. A major responsibility of the laser nurse or technician is to evaluate the progress of the laser operation and have the machine switched to standby when laser energy is not required. The phrase, “laser on,” spoken by the operating laser surgeon and required before the laser is activated, becomes as important as safety glasses, smoke evacuators, or the engineering of the machine itself in fostering safety. A team approach with the surgical laser technician, who basically is in charge of the laser, and the surgeon is essential.
Ignition of methane from the rectum or rumen can also be an exciting occurrence; the gas should first be removed by suction or blocked by tamponade. Vaporization of iodine skin preparations into irritating fumes, ignition of alcohol, or ignition of any pure oxygen environment mentioned previously are also important concerns.
4. Miscellaneous Problems
Other hazards include electrical injury from the high voltage power supply. Laser operation with newer devices is easy since they are extremely user-friendly and reliable, BUT machine maintenance including the purchase of maintenance contracts
may be required to maximize use and minimize safety concerns for mechanical, electrical, and optical failures. This aspect of medical laser usage must be recognized because maintenance contracts and laser repair can both be quite costly.
The use of Biomedical Lasers in Veterinary Medicine
Early reports concerning the use of lasers for medical applications involved animals, either as experimental models or as clinical veterinary patients. In 1968, the removal of a vocal-cord nodule in a dog demonstrated one of the first practical clinical applications of the carbon dioxide laser as a precision surgical instrument.28 Since that time, use of biomedical lasers has expanded tremendously in both small and large animal surgery. However, to some veterinarians, the laser is still a tool in search of an application. The rising popularity of the surgical laser has been influenced most often by their use in private practice and stems from a blend of its demonstrated precision and control, improved hemostasis, fewer signs of postoperative pain, increased client satisfaction, and affordability. An objective and practical approach to laser surgical procedures in veterinary medicine is essential if the total beneficial potential is to be realized. “Zap and vaporize” techniques coupled with a “burn and learn” philosophy can do potential harm to patient and operator and outweigh any beneficial effect. These concepts have no place in the objective use of lasers in medicine. A concerned effort must be made to evaluate the use of a laser for its potential patient benefit, rather than portraying it as a miracle device of the 21st century that is advertised on an illuminated bill board in front of a hospital. Although the use of biomedical lasers has created an entirely new definition for performing surgery, a surgeon’s knowledge of pathophysiology and technical expertise must be the primary factors to determine whether a laser should be used for a particular surgical procedure in lieu of more conventional approaches.4
Veterinary Clinical Applications– Small Animal
Many of the early reports involving the use of biomedical lasers concerned endoscopic use of fiber-delivered devices (Nd: YAG laser at 1064 nm) for treatment of laryngeal conditions and pathology of the upper respiratory system in the horse.17,29,30 Since that time, however, a number of investigators and many practitioners have used carbon dioxide, diode, and Nd: YAG lasers in the treatment of various surgical conditions in small animals.4,5,18,31-46 Most recently, use of the carbon dioxide laser for both excisional and ablative procedures has become common in many small animal practices. Well informed clients have often requested “laser surgery” due to extensive efforts towards marketing the technology by both veterinarians and laser manufacturers. Often, the procedure of choice for laser surgery has been a feline laser onychectomy.32 Results that include minimal intra-operative hemorrhage and decreased perception of post-operative pain have been the primary advantages. In addition, elective procedures including laser ovariohysterectomy and orchidectomy have also been promoted for similar reasons. Other applications in general surgery have included conventional soft tissue procedures where precise dissection and control of hemorrhage is important. These procedures have included liver biopsy, resection of hepatic lobes, splenic biopsy, prostatic dissection and ablation, partial nephrectomies and nephro- tomies, and excision/resection of a variety of intra abdominal, intrathoracic, cutaneous, and mammary neoplasms.31 Reports have reviewed clinical uses of laser energy for ablation/palliation of a brain tumor (Nd:YAG), ablation of neoplasms (CO2, Nd:YAG), and treatment of eosinophilic granulomas (CO2, Nd:YAG), perianal fistulas (Nd:YAG, CO2), or acral lick dermatitis (Nd:YAG, CO2).33,36,38,42,43,46 Upper airway surgery, especially excision of an elongated soft palate in the dog, is most easily performed using laser energy with minimal post-operative complications.41
With advantages of lower morbidity time for some conditions, less perceived signs of pain, and potential treatment regimes for conditions not amenable to conventional surgical/medical procedures, employment of biomedical lasers has not only found use in the clinical small animal setting, but also in the realm of exotic animal and avian practice, where even minimal blood loss can be significant in smaller patients. In addition, clinical use of the holmium:YAG laser for percutaneous prophylactic ablation of intervertebral discs and lithotripsy of urologic calculi in dogs have been reported and show tremendous potential.24,25,47-49 The use of biomedical lasers for veterinary ophthalmologic applications has been firmly established, although use has not become as common as it is in human medicine. The Q-switched or continuous wave ophthalmic Nd:YAG, argon, and diode lasers have been used as funduscopic photocoagulators in retinopathies, for treatment of lens-induced pupillary opacification, and for transcleral laser cyclodestruction of the ciliary body for glaucoma therapy in dogs. The carbon dioxide laser has also been used for soft tissue periocular and scleral surgical procedures. As experience and interest increases, and lasers become more available to veterinary ophthalmologists, clinical applications will increase as treatment protocols are initiated and proven useful. 18,50
Photodynamic therapy (PDT) has been used for clinical applications in veterinary medicine by several investigators. A number of initiatives have been reported using PDT for treatment of spontaneously occurring neoplasms in dogs and cats. This exciting treatment modality for selective destruction of neoplasms, employing interaction of a photosensitizer with light in the presence of oxygen, will continue to play a more dominant role in clinical veterinary medicine as protocols are established and new photosensitizing drugs are manufactured and approved for use. 19,51
Use of biomedical lasers in veterinary orthopedics has been more limited due to a lack of laser devices with appropriate wavelengths for incisional and ablative procedures in bone.52-54 The horse has been used as a model for biostimulation of articular cartilage and other research applications using the Ho: YAG laser.21 Practical use of lasers for ablation of bone has not been effective, although laser ablation (CO2) of methylmethacrylate during removal/revision of total hip prosthesis is possible.45
General Surgical Technique in Laser Surgery
The use of surgical lasers can be broadly classified as incisional or ablative surgery. For incisional surgery, a small spot size (0.2 to 0.4 mm) which delivers a high power density is ideal. The main reason surgical lasers are used for incisional surgery is because of the excellent degree of hemostasis obtained. At the tissue interface, blood vessels less than 0.5 mm in diameter can be coagulated and sealed so that use of the surgical laser as a light scalpel is relatively hemostatic in most capillary beds and in the transection of small venules and veins. Lymphatics are also sealed so postoperative edema may be minimized. Subjectively, there seems to be less pain associated with a laser incision and dissection. This observation could be due to the fact that smaller nerves are sealed or even spared at some laser wavelengths. 55 Microorganisms are also destroyed in the process of photothermal ablation, so tissues may be “disinfected” (bacterial numbers reduced by reduction of numbers due to direct vaporization) during laser tissue-interaction.57,58
The depth of the incision made by a surgical laser is both a function of the irradiance (power density) and the speed with which the incision is made. With practice, the surgeon can use the laser beam as precisely as the scalpel, with the added advantage of less hemorrhage, and less pain, although objective, published results in veterinary medicine are few.59,60 Laser incisions tend to be made more slowly than those made with a scalpel, at least initially. The improved hemostasis and incisional control generally makes up for this delay, and in some cases involving highly vascular tissue, a laser incision may actually make it possible to perform laser surgery faster than conventional surgery. Care must be taken not to create excessive collateral photothermal injury (char formation) during the process. Providing tissue counter tension during the incisional procedure aids not only tissue separation, as it does with a scalpel, but also decreases the amount of char formation. A defocused laser beam (holding the handpiece or cleaved optical fiber an appropriate distance from the tissue surface) can be used in some cases to stop bleeding from larger blood vessels that were not sealed by the focused or contact-mode incisional laser beam. Tissue excised with a surgical laser can still be histopathologically evaluated for tumor margins without much difficulty, if proper technique is used that minimizes collateral photothermal damage and the pathologist is informed that a laser was used for the biopsy.40 As mentioned earlier, healing of laser incisions is minimally delayed due to photothermal collateral tissue interaction.11,12,61
Tissue ablation or vaporization is most easily accomplished using a defocused or non-contact, free-beam mode of energy delivery. Defocused beam delivery through an articulated arm or a hollow waveguide can be utilized to ablate tissue efficiently, if carbonization (char formation) is minimized. To accomplish this, optical and mechanical scanners (described previously) are ideal accessories for the carbon dioxide laser. In addition, as char formation occurs, the surgeon should be diligent to remove any buildup of carbonized tissue by using saline moistened gauze sponges to mechanically debride the ablated tissue surface.
Tissue ablation can also be performed using fiberoptic delivery systems in non-contact mode with compatible laser wavelengths (diode – 808/980 nm; Nd:YAG to 1064 nm; Ho:YAG – 2100 nm). Laser power and energy delivery levels must be substantially higher (> 20 W < 100 W) for non-contact, free-beam tissue
ablation using fiber optic delivery. It must also be understood that a laser fiber used for contact mode delivery for incisional purposes cannot usually be immediately changed from contact mode to non-contact mode free-beam energy delivery. Since contact mode incisional surgery requires the fiberoptic tip to be carbonized so it can absorb adequate energy to incise tissue, higher energy levels required for non-contact ablation will usually melt the fiberoptic tip. Using a freshly cleaved, a surgeon can go from non-contact, free-beam energy delivery to contact delivery, but cannot go from contact laser surgery to non-contact delivery without re-cleaving the fiber. In the case of sculptured fiber tips (tapered, orb) meant to be used only in contact mode, high power free-beam delivery should be avoided to prevent premature fiber degradation. However, once a sculpted fiber tip is degraded, the fiber can be cleaved and reused in that configuration for both free-beam and contact delivery.
Future Innovations
The use of lasers in medicine is an exciting treatment modality that will continue to produce innovative and new methods for managing diseased tissue. Research focused on basic laser-tissue interaction and selective tissue destruction will become increasingly important. The use of photodynamic therapy (PDT) for treatment of malignant tumors will become an effective part of the veterinary oncologist’s armamentarium as more efficacious photosensitizers become available and expanded use of lower cost lasers or even non-laser light sources occurs. Photothermolysis using appropriate chromophores for selective tissue destruction and sterilization/disinfection is currently proving to be efficacious in both the clinical and laboratory settings. Minimally invasive urologic techniques for ablation of bladder, urethral, and prostatic pathologic conditions in small animals will become more common as technologically enhanced and smaller endoscopes are developed, as delivery systems are improved, and as new laser wavelengths are investigated. Laser lithotripsy is now possible using both visible and infrared wavelengths. This technology is currently being used in academic and specialty hospital settings permitting minimally invasive lithotripsy of urinary tract calculi. Tissue fusion/welding of blood vessels, alimentary tract, ureter or urethra, skin, and even bone will become clinically available in the near future. Application of lasers for micromanipulation of gametes and laser energy for improving fertilization and hatching rates during in vitro fertilization in domestic animals are close to becoming clinical realities. The use of lasers for soft tissue dental procedures is already feasible and, as investigations continue, use of laser energy for hard tissue dental procedures will be possible.
Low level laser therapy (LLLT), or biostimulation, is now being used commonly in a variety of therapeutic settings in veterinary medicine. The efficacious use of this modality to decrease inflammation and pain, as well as enhance wound healing will continue to be investigated. Well controlled studies are underway using reliable LLLT devices. Positive objective results will provide additional therapeutic option for the practitioner and rehabilitation specialists.63
Development of user-friendly, durable, portable, less expensive laser systems is definitely on the near horizon. Semiconductor laser development from ultraviolet to far infrared wavelengths is feasible. At this point in biomedical laser technology, diode laser development and similar technologies seem to hold the greatest promise. Use of lasers as diagnostic tools and sensors is one of the fastest growing branches of biomedical laser development. Clinical applications involving noninvasive recognition of malignant cells, abnormal tissue, or abnormal metabolites have tremendous potential. Use of available and future laser diagnostic technology could have a significant impact on the veterinary profession if a reasonable cost for equipment can be realized.
Future use of lasers in medicine depends on the active partici- pation of veterinarians in the inception and development of new devices that meet the needs of the entire medical profession. The sensible clinical approach that must be taken every day in the practice of veterinary medicine equips the veterinarian with a unique ability to understand the practical and economic values of biomedical lasers. Veterinary medicine can and should be in the forefront during these exciting times, adding an essential dimension to development of this 21st century technology.
References
- Swaim, CP, Mills, TN. A history of lasers. In: Krasner N, ed. Lasers in gastroenterology. New York: Wiley-Liss, 1991: 3.
- Katzir, A: Medical Lasers. In: Lasers and Optical Fiber in Medicine, Academic Press, Inc., San Diego, CA, 1993:15.
- Anderson, RR. Laser-tissue interactions in dermatology. In: Arndt, RA, ed. Lasers in cutaneous and aesthetic surgery. Philadelphia: Lippincott- Raven, 1997: 25.
- Bartels, K.E., Lasers in Veterinary Medicine – Where Have We Been, Where Are We Going, In Vet Clin Sm An Pract, W.B. Saunders, Philadelphia, PA, 2002; 32 (3): 495, 2002.
- Lucroy, MD, Bartels, KE. Surgical lasers. In: Slatter, D, ed. Textbook of Small Animal Surgery (3rd ed.). Philadelphia: Saunders, 2003: 227.
- Lucroy, MD, Magne, ML, et al. Low intensity laser light-induced closure of a chronic wound in a dog. Vet Surg, 1999; 28:292.
- Peavy, GM, Lasers and laser-tissue interaction, In: Bartels, KE. ed. Vet Clin NA: Laser in medicine surgery. WB Saunders, Philadelphia, 2002, 32(3): 517.
- Welch, AJ, van Gamert, MJC: Introduction to medical applications. In Welch, AJ, van Gamert, MJC (eds): Optical-thermal Response of Laser-irradiated Tissue, Plenum Press, New York, 1995: 609.
- Lubatschowski, H, Heistrkamp, A, Will, F., et al. Medical applications for ultrafast lasers. RIKEN Review No. 50: Focused on laser precision microfabrication, January, 2003, 113.
- Jacques, SL: Laser-tissue interactions. Photochemical, photo- thermal, and photomechanical. In Schwesinger, WH, Hunter, JG, (eds) Surg Clin NA: Lasers in general surgery. WB Saunders, Philadelphia, 1992; 72:531.
- Lopez, AP, Phillips, TJ. Wound healing. Wound healing. In: Fitzpatrick, RE, Goldman, MP (eds.) Cosmetic Laser Surgery. St. Louis: Mosby, 2000: 31.
- Taylor, DL, Schafer, SA, Nordquist, et al: Comparison of a high power diode laser with the Nd: YAG laser using in situ wound strength analysis of healing cutaneous incisions. Lasers Surg Med, 1991; 21:248.
- Treat, MR, Oz, MC, Bass, LS. New technologies and future applications of surgical lasers. The right tool for the right job. In: Schwesinger, WH, Hunter, JG, eds. Surg Clin NA: Lasers in general surgery. WB Saunders, Philadelphia, 1992, 72: 705 - 747.
- Hecht, J: Carbon dioxide lasers. In The Laser Guidebook, New York, McGraw-Hill, 1992: 159.
- Judy, MM, Matthews, JL, Aronoff, BL, Hults, DF. Soft tissue studies with 805 nm diode laser radiation: Thermal effects with contact tips and comparison with effects of 1064 nm Nd: YAG laser radiation. Lasers Surg Med, 1993, 13: 528.
- Katzir, A: Single optical fibers. In Lasers and Optical Fibers in Medicine, Academic Press, Inc., San Diego, CA, 1993:107.
- Tullners, EP: Transendoscopic contact neodymium: yttrium aluminum garnet laser correction of epiglottic entrapment in standing horses. JAVMA, 1990; (144): 1971.
- Gilmour, MA. Lasers in ophthalmology. In: Bartels, KE. ed. Vet Clin NA: Laser in medicine surgery. WB Saunders, Philadelphia, 2002, 32(3): 649.
- Lucroy, MD, Photodynamic therapy for companion animals with cancer, In: Bartels, KE. ed. Vet Clin NA: Laser in medicine surgery. WB Saunders, Philadelphia, 2002, 32(3): 693.
- May, K.A., Pleasant, R.S., Howard, R.D., Moll, H.D., Duesterdieck, K.F., MacAllister, C.G. and Bartels, K.E., Failure of Holmium: yttrium-aluminum Garnet Laser Lithotripsy in Two Horses with Calculi in the Urinary Bladder, JAVMA, 2001, 219:957.
- Collier, M, Haugland, LM, Bellamy, J, et al: Effects of holmium: YAG laser on equine articular cartilage and subchondral bone adjacent to traumatic lesions: a histopathological assessment, J Arthroscopic and Related Surg1997, 9: 536.
- Teichman, JM, Vassar, GJ, Glickman, RD, Holmium: yttrium-aluminum-garnet lithotripsy efficiency varies with stone composition. Urology 1998; 52: 392.
- Bartels, K.E., Higbee, R.G., Bahr, R.J., Galloway, D.S., Healey, T.S., Arnold, C.S., Outcome of and Complications Associated with Prophy- lactic Percutaneous Laser Disk Ablation in Dogs with Thoracolumbar Disk Disease: 277 cases (1992-2001), JAVMA, 2003; 222: 1733.
- Dickey, DT, Bartels, KE, Henry, et al: Use of the holmium yttrium aluminum garnet laser for percutaneous thoracolumbar intervertebral disk ablation in the dog paper, JAVMA, 1996; 208: 1263.
- Sliney, DH. Laser Safety. Lasers Surg Med, 1995; 16: 215.
- Fry, TR: Laser safety, In: Bartels, KE. ed. Vet Clin NA: Laser in medicine surgery. WB Saunders, Philadelphia, 2002, 32(3): 535.
- Jako, GJ: Laser surgery of the vocal cords; an experimental study with the carbon dioxide laser on dogs, Laryngoscope, 1972; 82: 2204.
- Sullins, KE. Diode laser and endoscopic laser surgery. In: Bartels, KE. ed. Vet Clin NA: Laser in medicine surgery. WB Saunders, Philadelphia, 2002, 32(3): 639.
- Tate, LP, Sweeney, CL, Bowman, KF, et al: Transendosocpic Nd: YAG laser surgery for treatment of epiglottal entrapment and dorsal displacement of the soft palate in the horse. Vet Surg, 1990; 19: 356.
- Holt, TL, Mann, FA, Soft tissue application of lasers. In: Bartels, KE. ed. Vet Clin NA: Laser in medicine surgery. WB Saunders, Philadelphia, 2002, 32(3): 569.
- Young, WP. Feline onychectomy and elective procedures. In: Bartels, KE. ed. Vet Clin NA: Laser in medicine surgery. WB Saunders, Philadelphia, 2002, 32(3): 601.
- Shelley, BA. Use of the carbon dioxide laser for perianal and rectal surgery. In: Bartels, KE. ed. Vet Clin NA: Laser in medicine surgery. WB Saunders, Philadelphia, 2002, 32(3): 621.
- Bellows, J. Laser use in veterinary dentistry. In: Bartels, KE. ed. Vet Clin NA: Laser in medicine surgery. WB Saunders, Philadelphia, 2002, 32(3): 673.
- Rupley, AE, Parrott-Nenezian, T. The use of surgical lasers in exotic and avian practice. In: Bartels, KE. ed. Vet Clin NA: Laser in medicine surgery. WB Saunders, Philadelphia, 2002, 32(3): 535.
- Hardie, EM, Stone, EA, Spaulding, KA, Cullen, JM. Subtotal canine prostatectomy with the neodymium: yttrium aluminum garnet laser. Vet Surg, 1990; 19: 348.
- Feder, BM, Fry, TR, Kostolich, M, Bartels, KE, Bahr, R, Mandsager, R, et al. Nd: YAG laser cytoreduction of an invasive intracranial menin- gioma in a dog. Prog Vet Neuro, 1993; 4:3.
- Ellison, GW, Bellah, JR, Stubbs, WP, Van Gilder, J. Treatment of perianal fistulas with Nd: YAG laser. Results in twenty cases. Vet Surg, 1995; 24:140.
- Nasisse, MP, Davidson, MG, English, RV, Jamieson, V, Harling, DE, Tate, LP. Treatment of glaucoma by use of transcleral neodymium: yttrium aluminum garnet laser cylcocoagulation in dogs. JAVMA, 1990; 197:350.
- Rizzo, L.B., Ritchey, J.W., Higbee, R.G., Bartels, K.E., Lucroy, M.D., Histologic Comparison of Skin Biopsy Specimens Collected by Use of Carbon Dioxide or 810-nm Diode Lasers from Dogs, JAVMA, 2004; 225:1562.
- Davidson, E.B., Davis, M.S., Campbell, G.A., Williamson, K.K., Payton, Healey, T.S., and Bartels, K.E.: Evaluation of Carbon Dioxide Laser and Conventional Incisional Techniques for Resection of Soft Palates in Brachycephalic Dogs. JAVMA, 2001; 219:776.
- Ellison, GW, Bellah, JR, Stubbs, WP, et al: Treatment of perianal fistulas with Nd:YAG laser. Results in twenty cases. Vet Surg, 1995; 24:140.
- Feder, BM, Fry, TR, Kostolich, et al: Nd:YAG laser cytoreduction of an invasive intracranial meningioma in a dog. Prog Vet Neuro, 1993, 4: 3.
- Hardie, EM, Carlson, CS, Richardson, DC: Effect of Nd: YAG laser energy on articular cartilage healing in the dog. Lasers Surg Med, 1989; 9: 595.
- Lange, DN, Rochat, MC, Bartels, KE, et al: Comparison of carbon dioxide laser modalities for removal of polymethylmethacrylate cement. Vet Comp Ortho Traum, 1997, 10: 25.
- Shelley, BA, Bartels, KE, Ely, RW, et al: Use of the neodymium: yttrium aluminum garnet laser for treatment of squamous cell carcinoma of the nasal planum in a cat. JAVMA, 1992; 201:756.
- Spindel, ML, Moslem, A, Bhatia, KS, Jassemnejad, B, Bartels, KE, Powell, RC, et al. Comparison of holmium and flashlamp pumped dye lasers for use in lithotripsy of biliary calculi. Lasers Surg Med, 1992; 12: 482.
- Davidson, E.B., Ritchey, J.W., Higbee, R.D., Lucroy, M.D., and Bartels, K.E., Laser Lithotripsy for Treatment of Canine Uroliths, Vet. Surg., 2004; 33:56.
- Wynn, V.M., Davidson, E.B., Higbee, R.G., Ritchey, J.W., Ridgway, T.D., Bartels, K.E., Lucroy, M.D., In Vitro Effects of Pulsed Holmium Laser Energy on Canine Uroliths and Porcine Cadaveric Urethra, Lasers Surg. Med., 2003; 33:243.
- Sapienza, JS, Miller, TR, Gum, GG, Gelatt, KN. Contact transcleral cyclophotocoagulation using a neodymium: yttrium aluminum garnet laser in normal dogs. Prog Vet Comparative Opthalmol, 1993; 2: 147.
- Klein, MK, Roberts, WG. Recent advances in photodynamic therapy. Compendium - Sm An, 1993; 15: 809.
- Roth, JE, Nixon, AJ, Gantz, VA: Pulsed carbon dioxide laser for cartilage vaporization and subchondral bone perforation in horse, Part I: Technique and clinical results. Vet Surg, 1991, 203(3): 190.
- Nixon, AJ, Krook LP, Roth JE, et al: Pulsed carbon dioxide laser for cartilage vaporization and subchondral bone perforation in horses. Part II: Morphologic and histochemical reactions. Vet Surg, 1991, 203 (3): 200.
- Peavy, GM, Reinisch, L, Payne, JT. Comparison of cortical bone ablations by using infrared laser wavelengths 2.9 to 9.2 micron, Lasers Surg Med 1999; 25(5): 421.
- Haugland, LM, Collier, MA, Panciera, RJ, Bellamy, J. The effect of CO2 laser neurectomy on neuroma formation and axonal regeneration. Vet Surg, 1992, 21(5): 351.
- Montgomery, TC, McNaughton, SD. Investigating the CO2 laser for plantar digital neurectomy in horses, Lasers Surg Med. 1995; 5(5):515.
- Shultz, R, Cabello F, Harvey G. Bacterial side effects of neodymium YAG lasers. Lasers Surg Med, 1986; 6:162.
- Hooks, WT: Use of CO2 laser sterilization. Oral Surg, 1980; 49:263.
- Mison, MB, Bohart, GH, Walshaw, R, et al. Use of carbon dioxide laser for onychectomy in cats JAVMA 2002; 221(5): 651.
- Palesty, JA, Zahir, KS, Dudrick, SJ, Ferri, S, Tripodi, G. Nd: YAG laser surgery for the excision of pilonidal cysts: a comparison with traditional techniques. Lasers Surg Med. 2000, 26(4):380.
- Mison, MB, Steicek, B, Lavagino, M, et al. Comparison of the effects of the CO surgical lasers and conventional surgical techniques on the healing and wound tensile strength of skin flaps in the dog, Vet Surg 2003; 32(2): 153.
- Irwin, JR: The economics of surgical laser technology in veterinary practice, In: Bartels, KE. ed. Vet Clin NA: Laser in medicine surgery. WB Saunders, Philadelphia, 2002, 32(3): 559.
- Aukri, R, Lubort, R, Taitel baum: Estimation of optimal wave lengths for laser-induced wound healing, Laser Surg Med. 2010, 42(8): 760.
Get access to all handy features included in the IVIS website
- Get unlimited access to books, proceedings and journals.
- Get access to a global catalogue of meetings, on-site and online courses, webinars and educational videos.
- Bookmark your favorite articles in My Library for future reading.
- Save future meetings and courses in My Calendar and My e-Learning.
- Ask authors questions and read what others have to say.
About
How to reference this publication (Harvard system)?
Author(s)
Copyright Statement
© All text and images in this publication are copyright protected and cannot be reproduced or copied in any way.Related Content
Readers also viewed these publications
Buy this book
Buy this book
This book and many other titles are available from Teton Newmedia, your premier source for Veterinary Medicine books. To better serve you, the Teton NewMedia titles are now also available through CRC Press. Teton NewMedia is committed to providing alternative, interactive content including print, CD-ROM, web-based applications and eBooks.
Teton NewMedia
PO Box 4833
Jackson, WY 83001
307.734.0441
Email: [email protected]






Comments (0)
Ask the author
0 comments